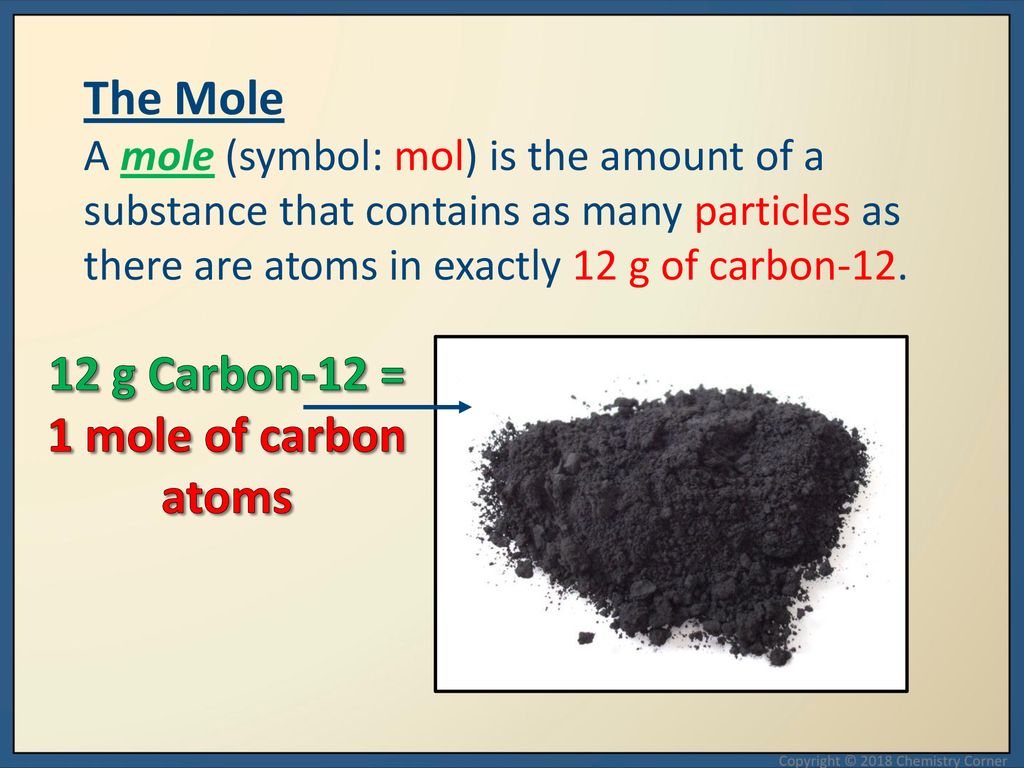
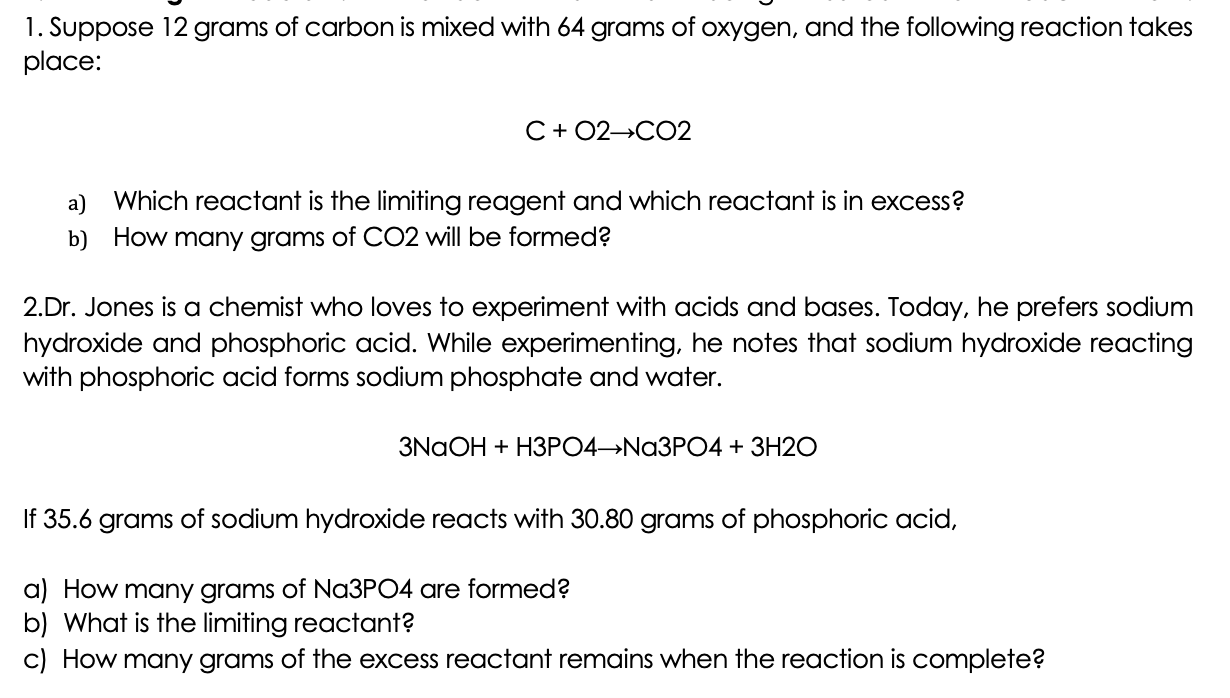
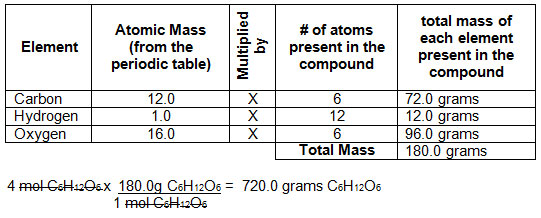
12 g C - 12 contains 6.022 × 10^23 atoms of carbon.(a) 6.022 × 10^23 is known as .............(b) Calculate the number of carbon atoms present in 48 g C - 12.(c)

If one mole of carbon atoms weighs 12 grams, what is the mass (in grams) of 1 atom of carbon?... - YouTube







![Term 2] Approximately how old is a fossil with 12 g of Carbon -14 if Term 2] Approximately how old is a fossil with 12 g of Carbon -14 if](https://d1avenlh0i1xmr.cloudfront.net/769024a3-6bed-4308-8ee9-7b70718b75e4/question-12d(choice-2).jpg)