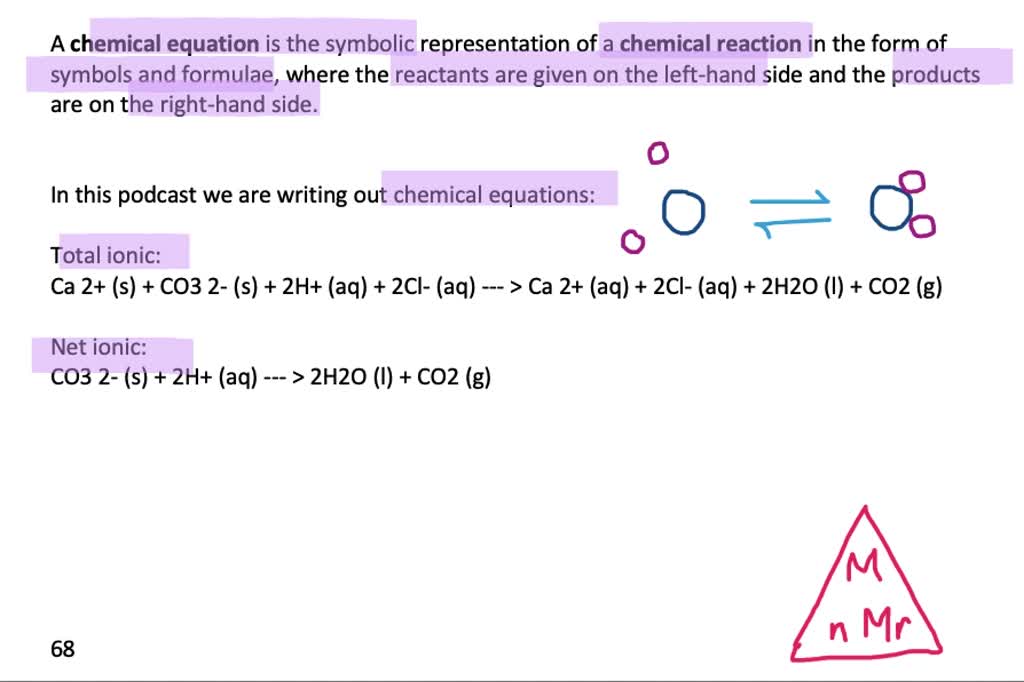
XXVI.—The reaction between calcium carbonate and chlorine water - Journal of the Chemical Society, Transactions (RSC Publishing)

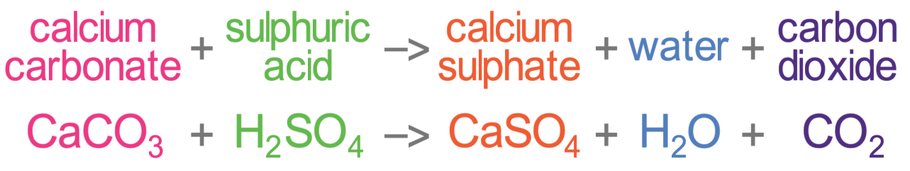
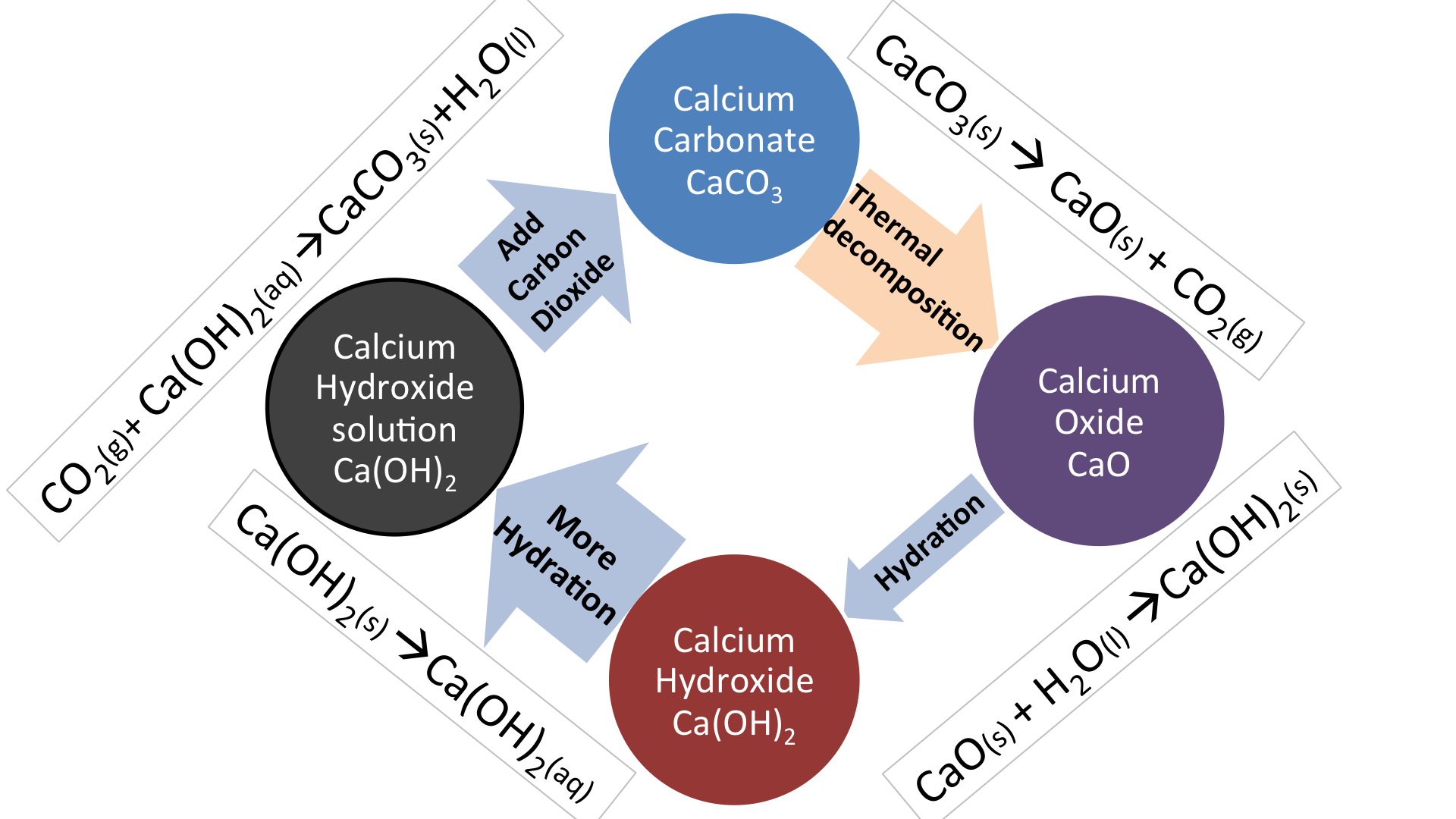
Write the balanced chemical equations for the following reactions.A Calcium hydroxide + Carbon dioxide → Calcium carbonate + waterB Zinc + Silver nitrate → Zinc nitrate + SilverC Aluminium + copper chloride
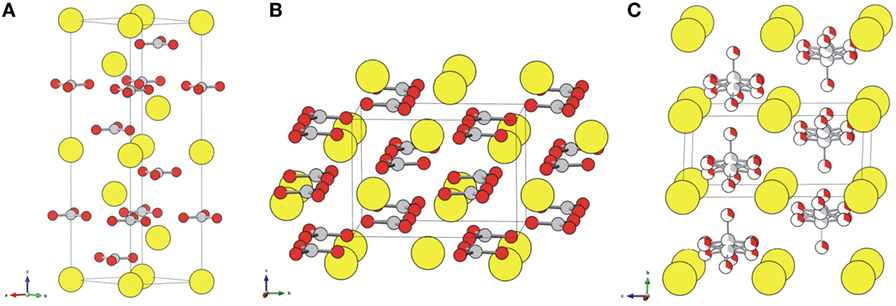
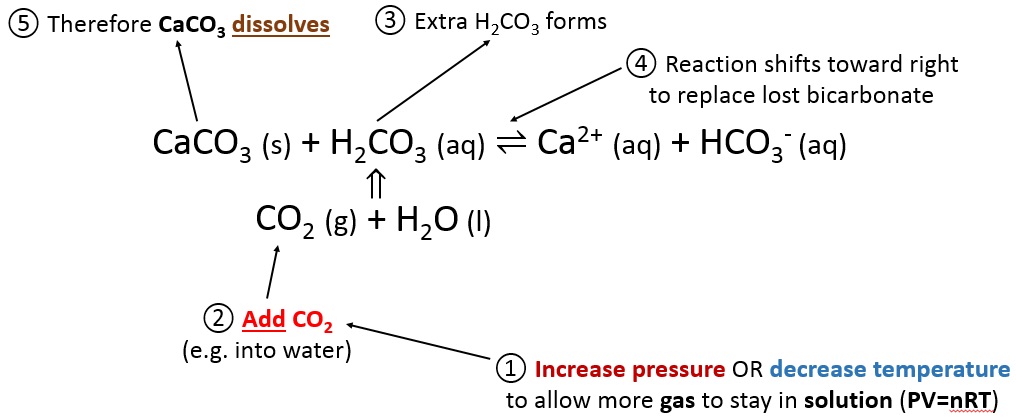
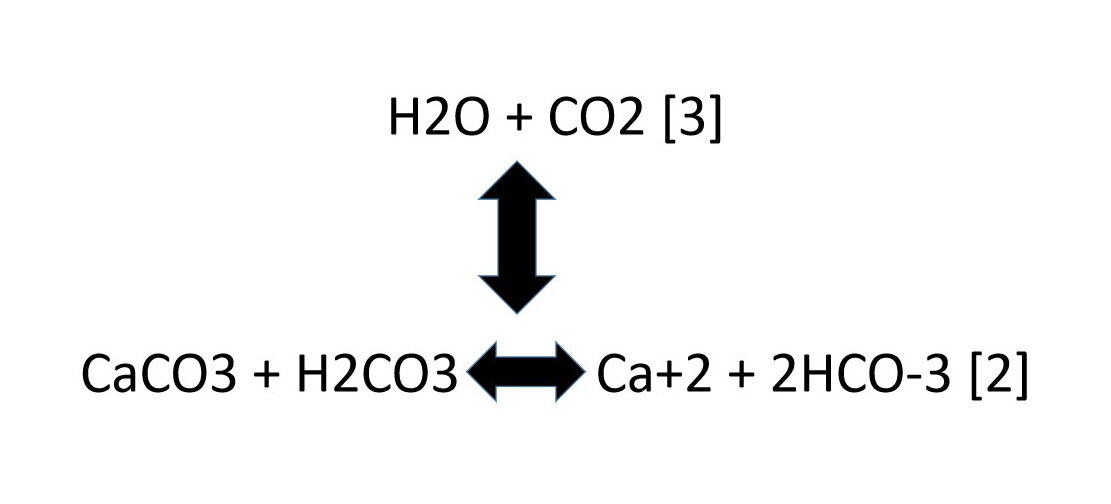
Frontiers | Calcium Carbonate Precipitation for CO2 Storage and Utilization: A Review of the Carbonate Crystallization and Polymorphism

Summary of the reactions between carbon dioxide (CO2) with water (H2O)... | Download Scientific Diagram
Write the balanced chemical equations for the following reactions: i. Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water - Sarthaks eConnect | Largest Online Education Community