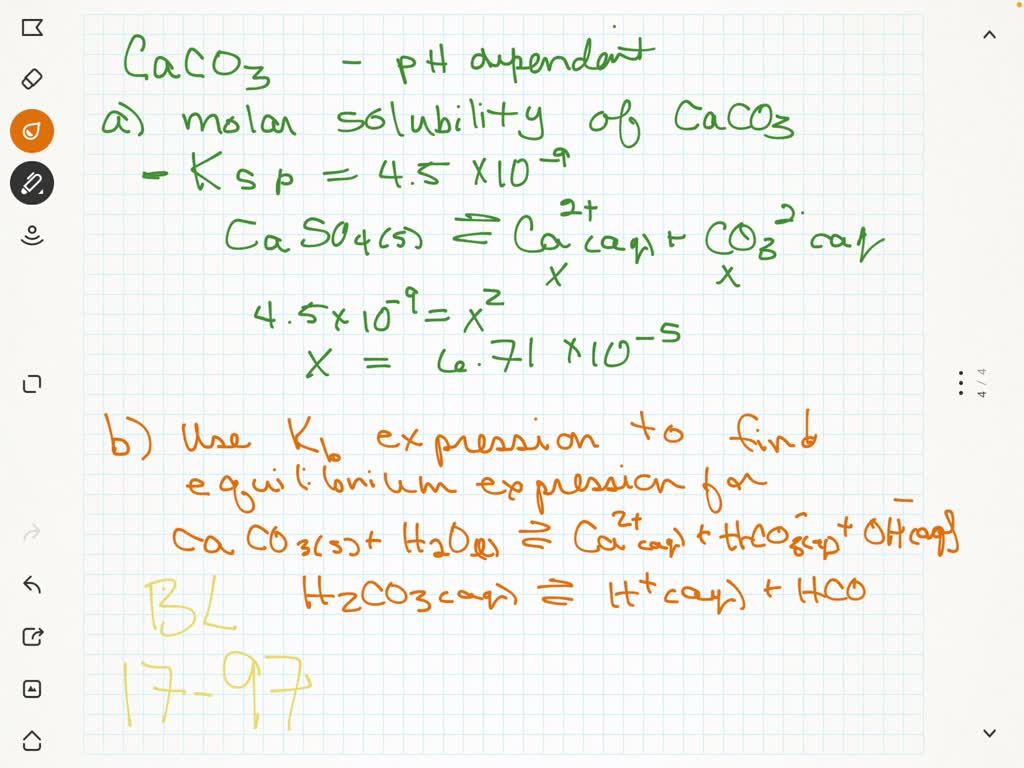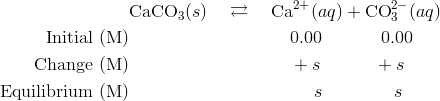The values of Ksp of CaCO3 and CaC2O4 are 4.7 x 10^-9 and 1.3 x 10^-9 respectively at 25°C. - Sarthaks eConnect | Largest Online Education Community
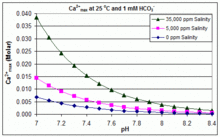
the solubility of CaCO3 is 7*10^ 3 mg/litre.calculate the solubility of BaCO3 (in mol/l) from this information and from the fact that when Na2CO3 is added slowly to a solution containing equimolar
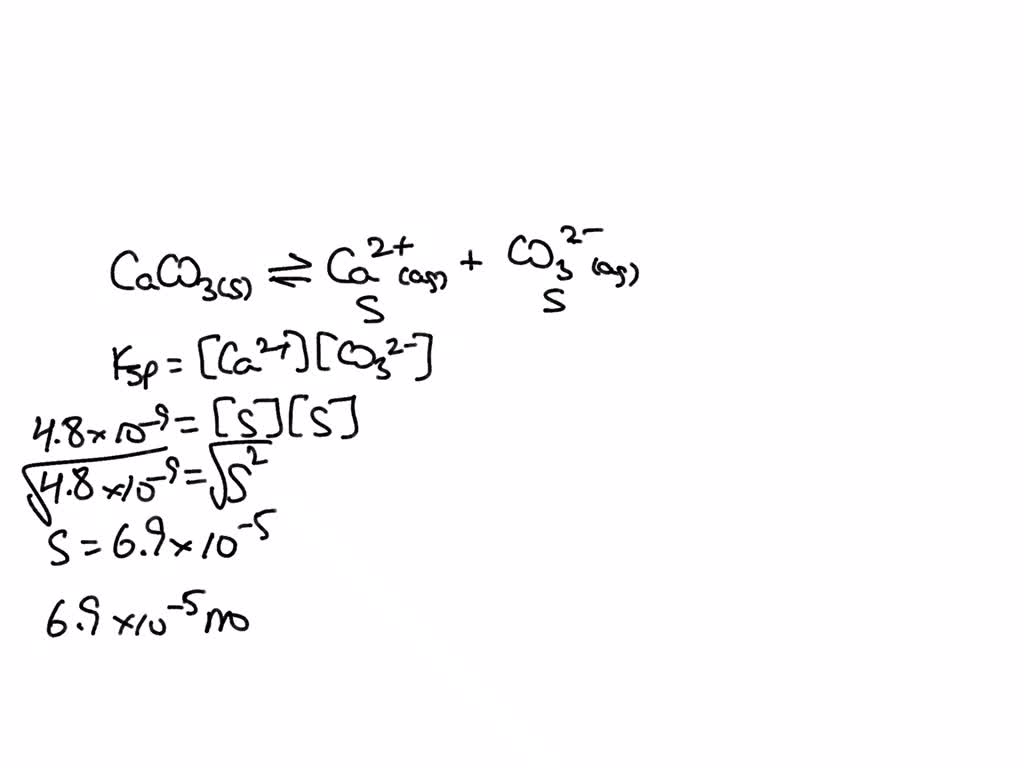
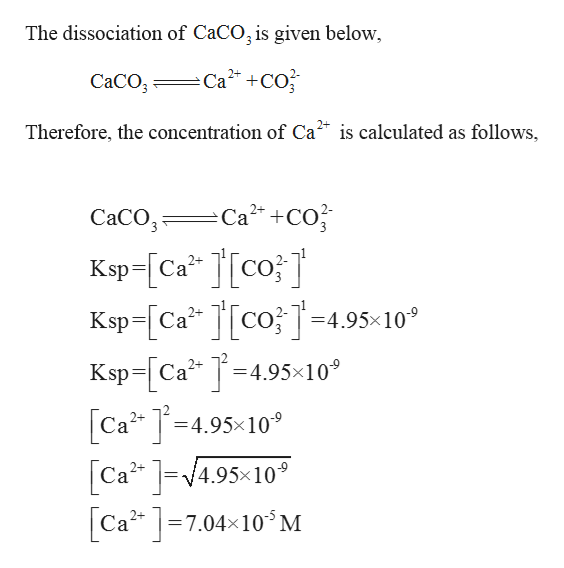
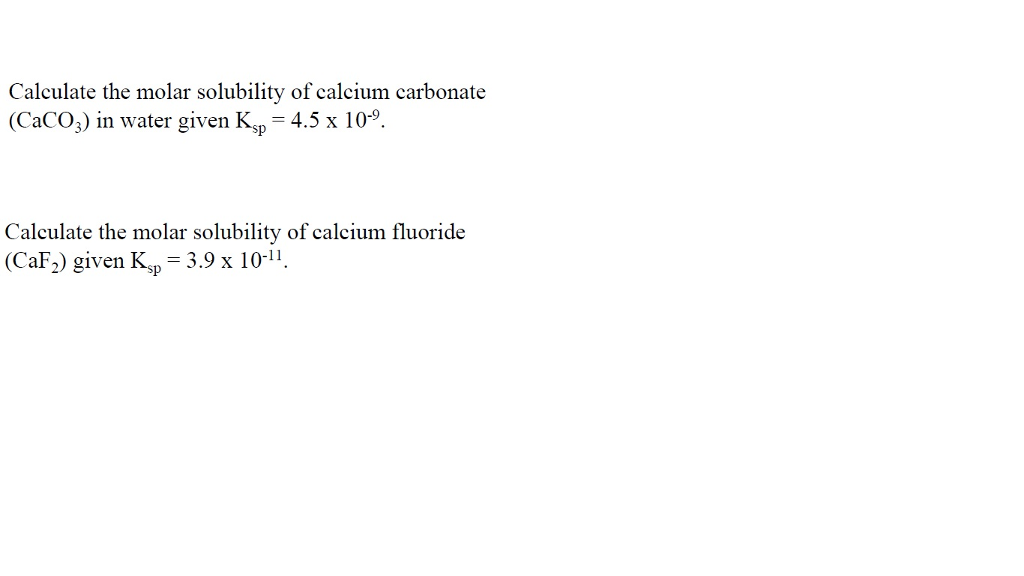
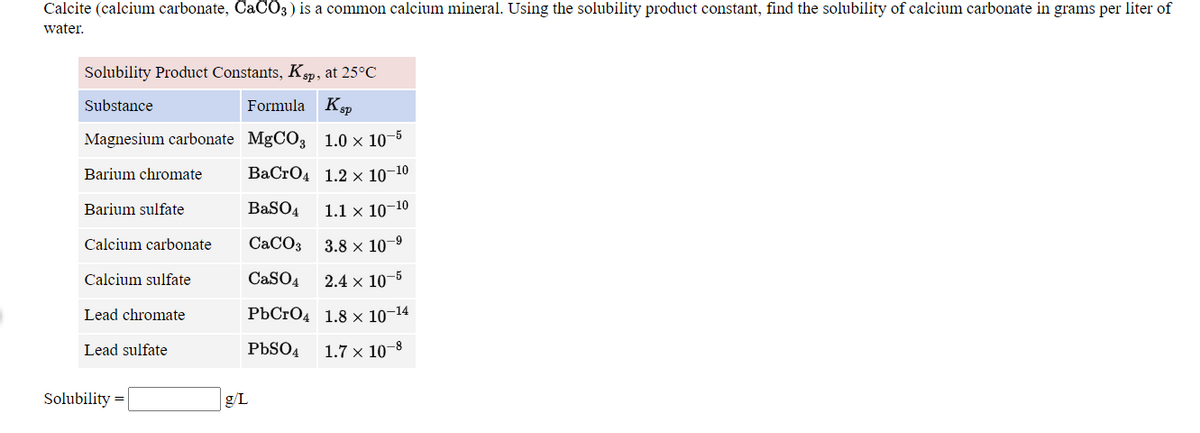
SOLVED: Calculate the solubility of calcium carbonate (CaCO3) in g / L. (Kps, CaCO3 = 4.8x10 ^ -9) (Mm, CaCO3 = 100.9 g / mol)A) 4.8x10^-10B) 6.9x10^-5C) 4.8x10^-9D) 6.9x10^-3

Factors controlling and influencing polymorphism, morphology and size of calcium carbonate synthesized through the carbonation route: A review - ScienceDirect
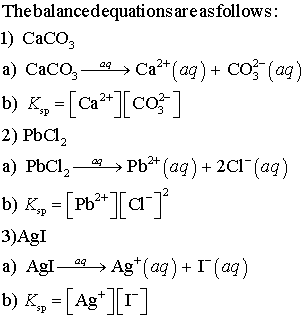
Write a balanced equation for the dissolution of CaCO3.Write an expression for Ksp for the dissolution - Home Work Help - Learn CBSE Forum

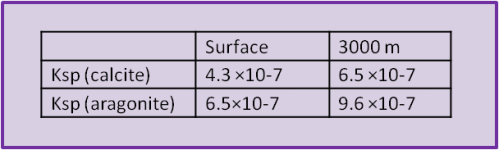
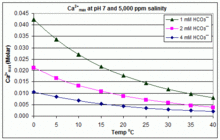
Solubility product (log_KSP) of various Ca-carbonates for temperatures... | Download Scientific Diagram
The values of K_sp of CaCO_3 and CaC_2O_4 are 4.7×10^ 9 and 1.3×10^ 9 respectively at 25^° C . If the mixture of these two is washed with water, what is the