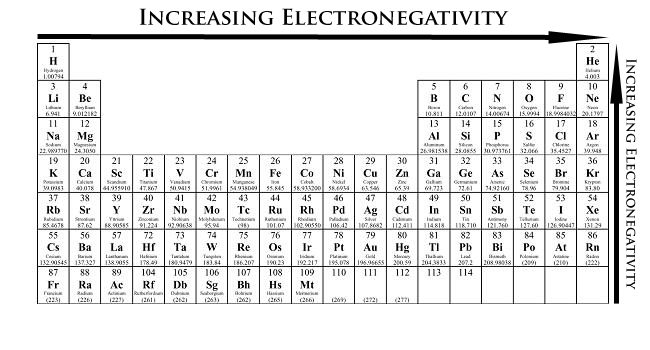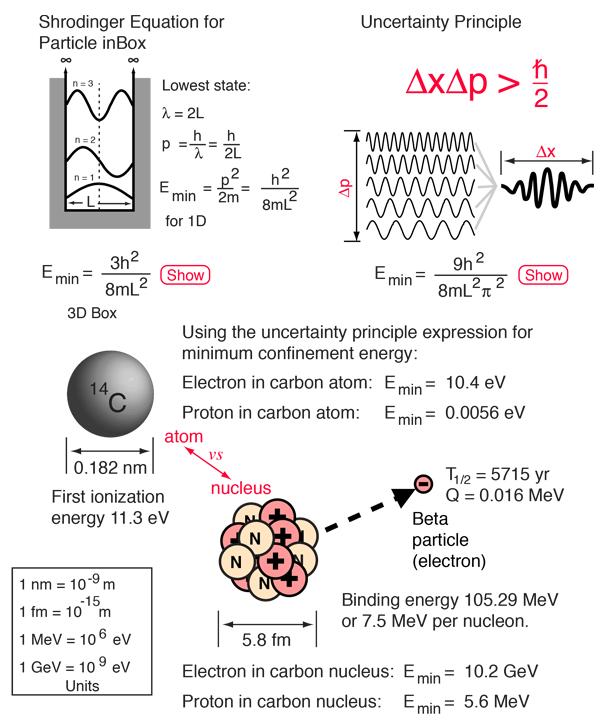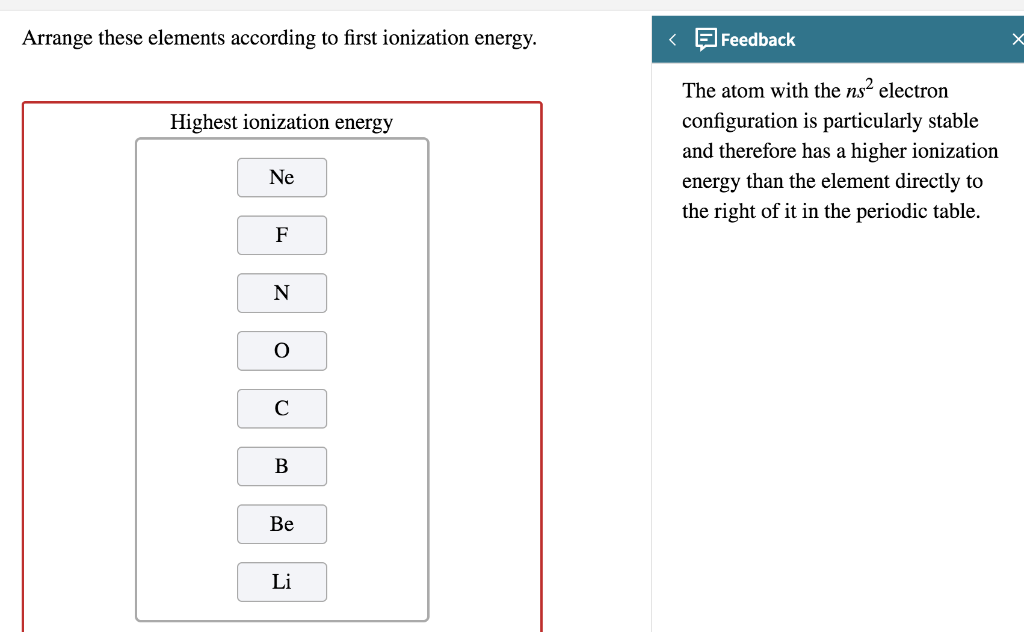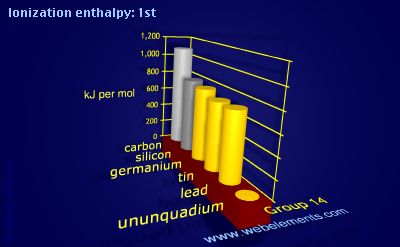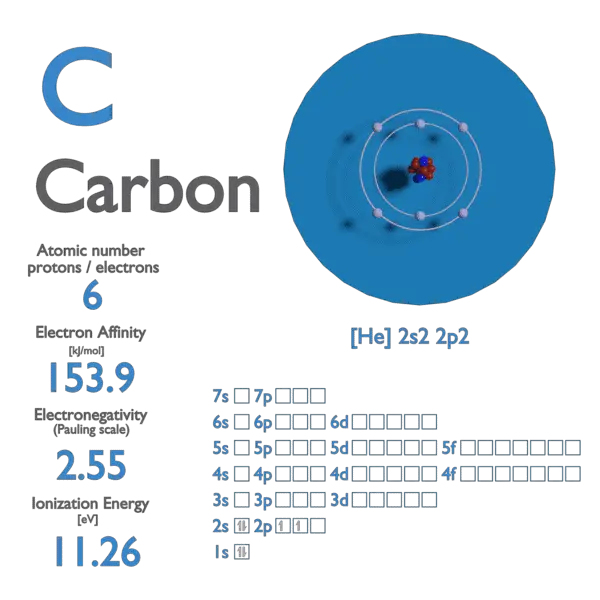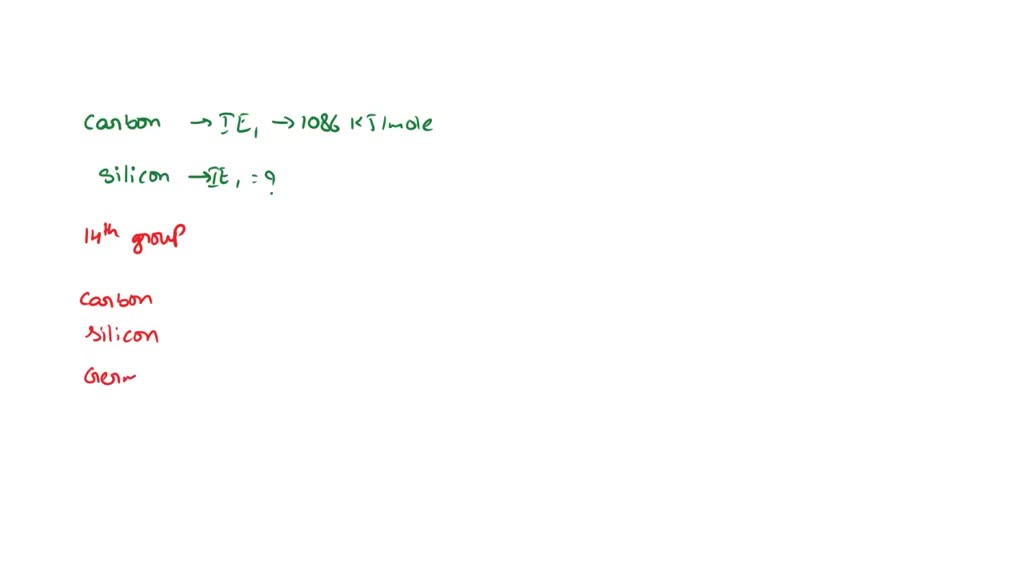
SOLVED: the first ionization energy for carbon is 1086 kJ/mol, then the first ionization energy for silicon will be a) 1086 kJ/mol. c) greater than 1086 kJ/mol. b) close to zero. d)

Can someone explain to me why Cl isn't the right answer? The top picture is the books explanation but I feel like Cl would have a higher ionization energy than carbon :

The first ionization energy of carbon is more than boron, but the second ionization energy is in reverse. Why? - Quora

Difference Between First and Second Ionization Energy (I1E vs I2E) | Compare the Difference Between Similar Terms