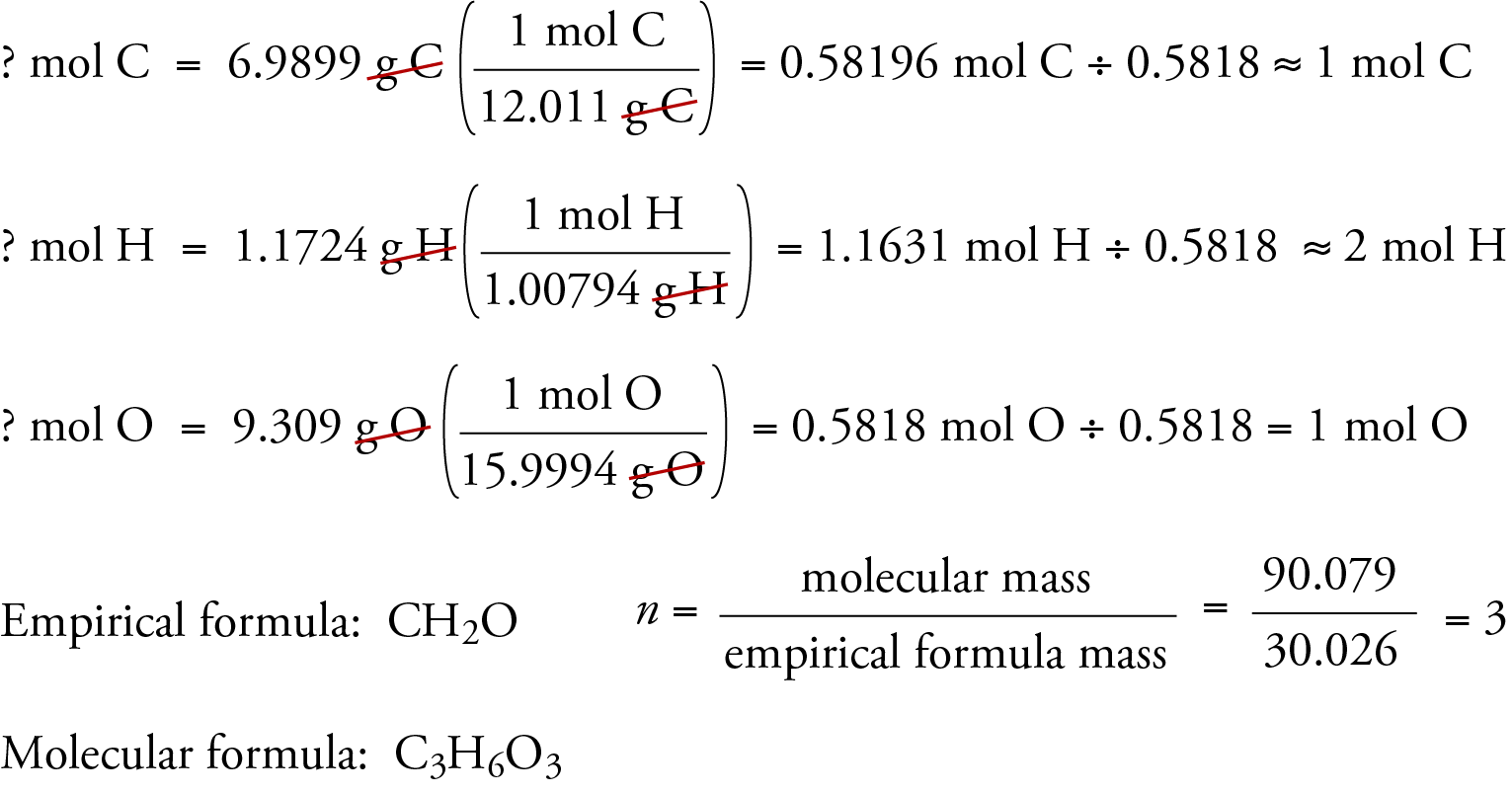
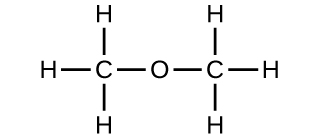
The empirical formula of a compound is CH2O and its vapour density is 30 . The molecular formula of the compound is :

SOLVED: A compound contains, by mass, 48.64% carbon, 8.16% hydrogen and 43.209 oxygen The empirical formula of this compound is: CzHsOz CaHgO3 CzH;O CzH;O

An organic compound contains carbon, hydrogen and oxygen. If the ratio percentage of C and - YouTube

3 A compound containing Carbon, Hydrogen and Oxygen gave the following analytical data: Carbon=40 0% , Hydrogen =6 67% - Chemistry - Some Basic Concepts of Chemistry - 12771131 | Meritnation.com
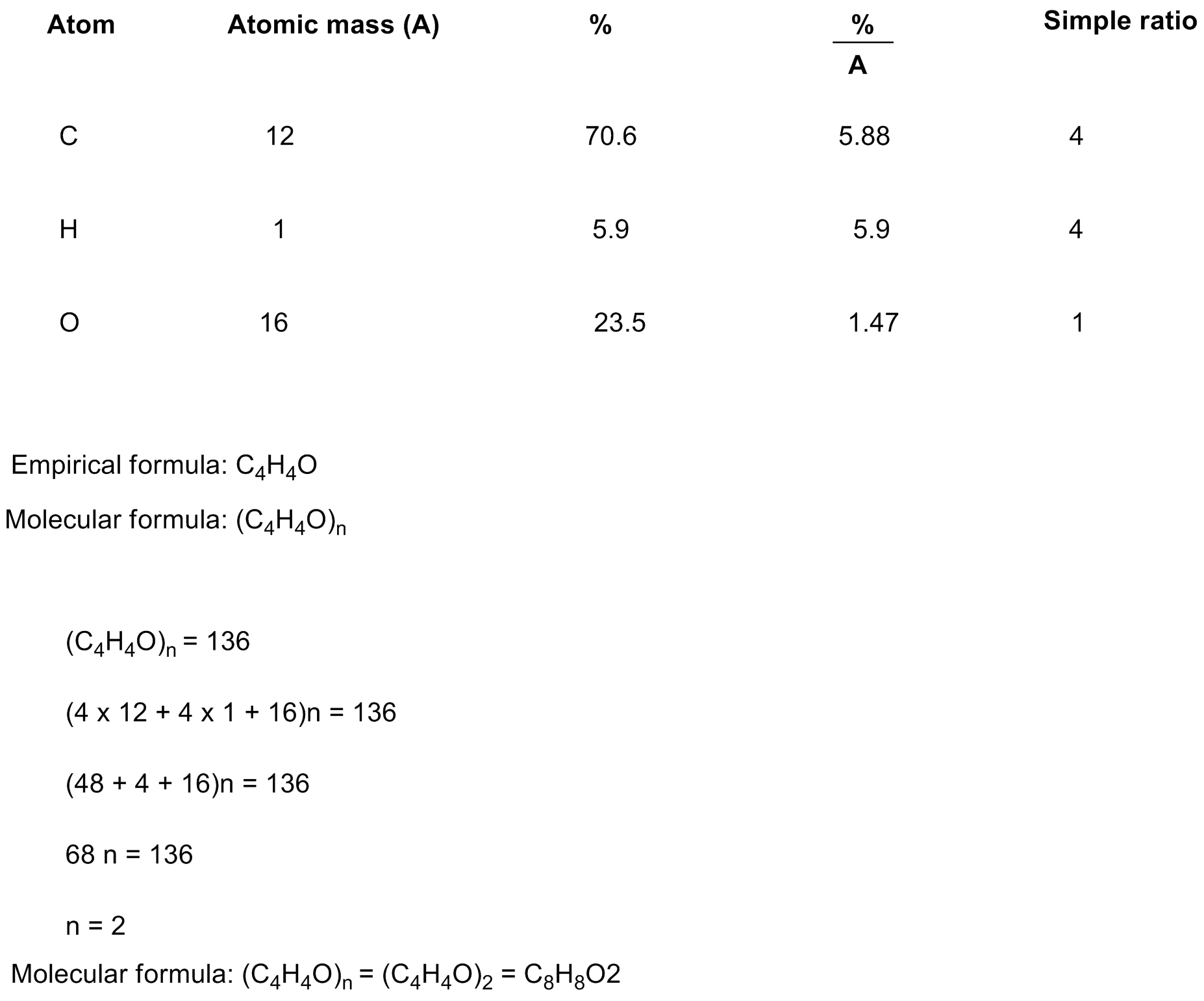
41) A compound that is composed of carbon, hydrogen, and oxygen contains 70.6% C, 5.9% H, and 23.5% O by mass. The molecular weight of the compound is 136 amu. What is
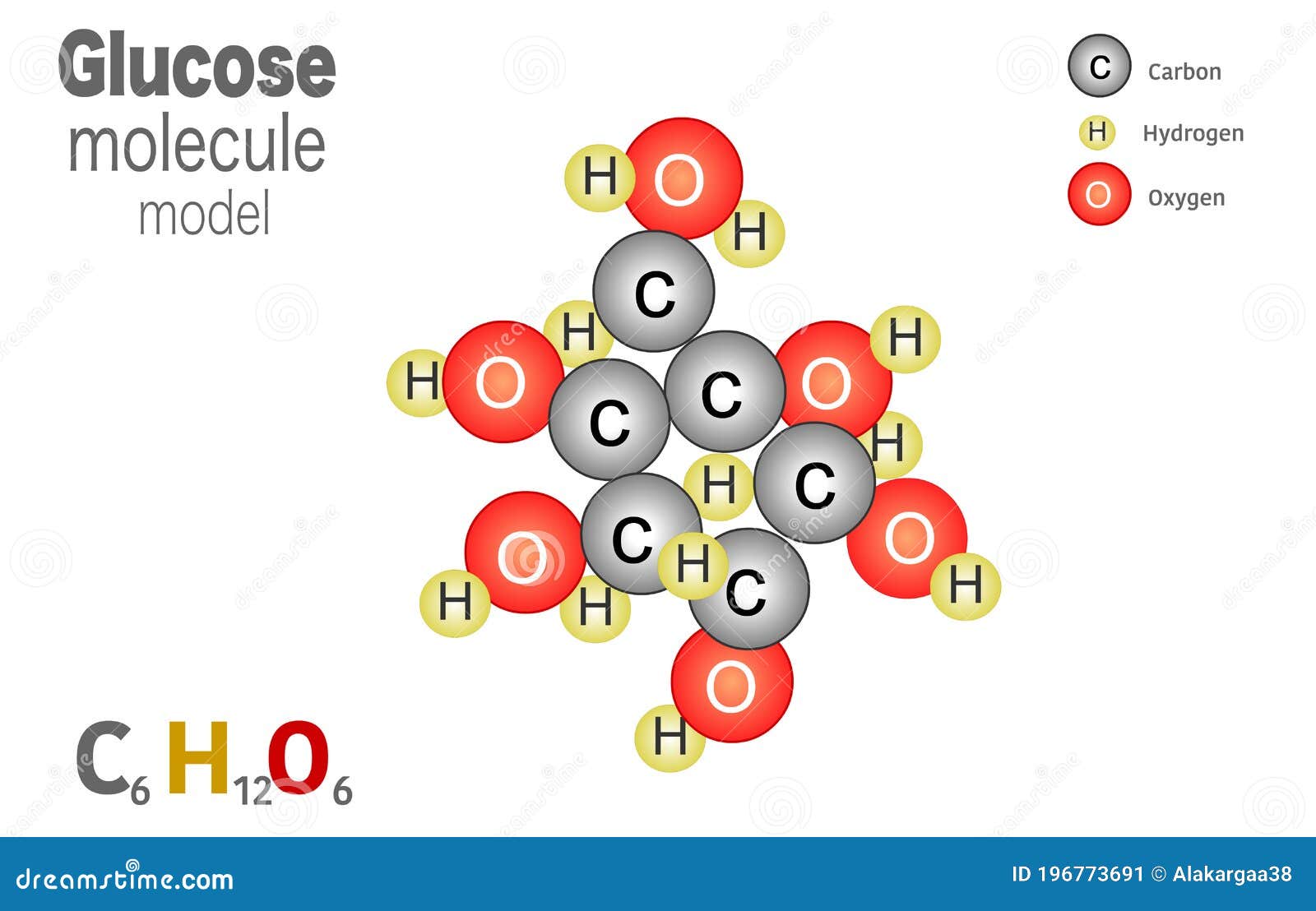
Glucose Molecule Model, Molecule is Formed from 6 Carbon Atoms, 12 Hydrogen Atoms and 6 Oxygen Atoms Linked Together. Molecular Stock Vector - Illustration of education, lesson: 196773691
.png)
an organic compound containing carbon ,hydrogen and oxygen gave the following composition : C- 54 55% ,H 9 09% and - Chemistry - - 9449851 | Meritnation.com

A compound containing only carbon, hydrogen and oxygen was analyzed and found to contain 3.25 % hydrogen and 19.36 % carbon. What is the empricial formula of the compound? | Homework.Study.com