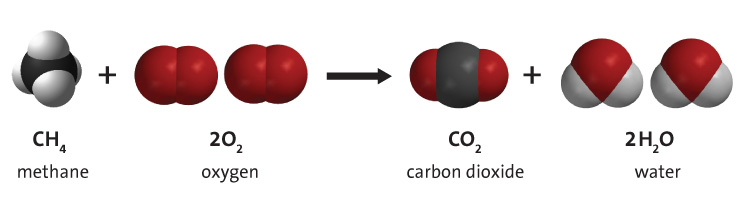
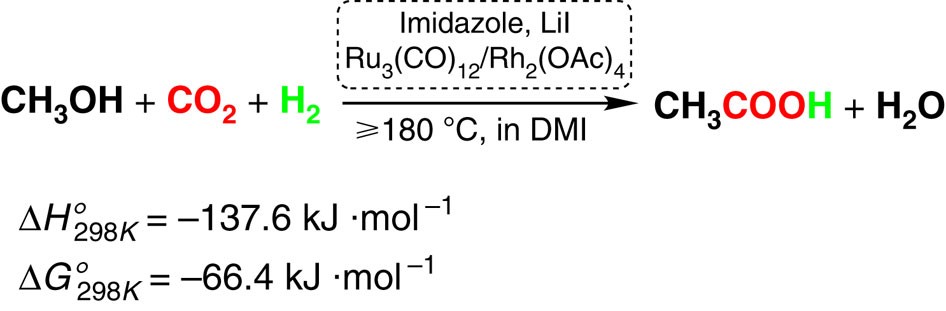
Alcohol-Assisted Hydrogenation of Carbon Monoxide to Methanol Using Molecular Manganese Catalysts | JACS Au

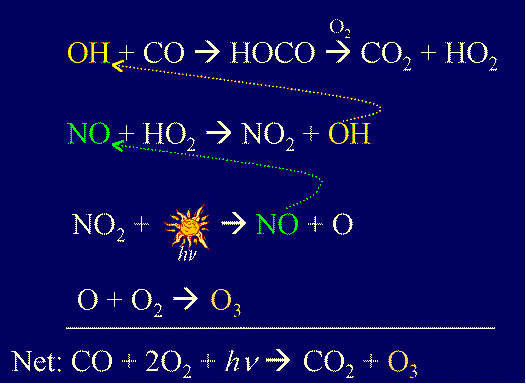
The enthalpies of formation of CO and `CO_(2)` are `-110.5 KJ mol^(-1)` and `-393.5 KJ mol^(-1)` - YouTube

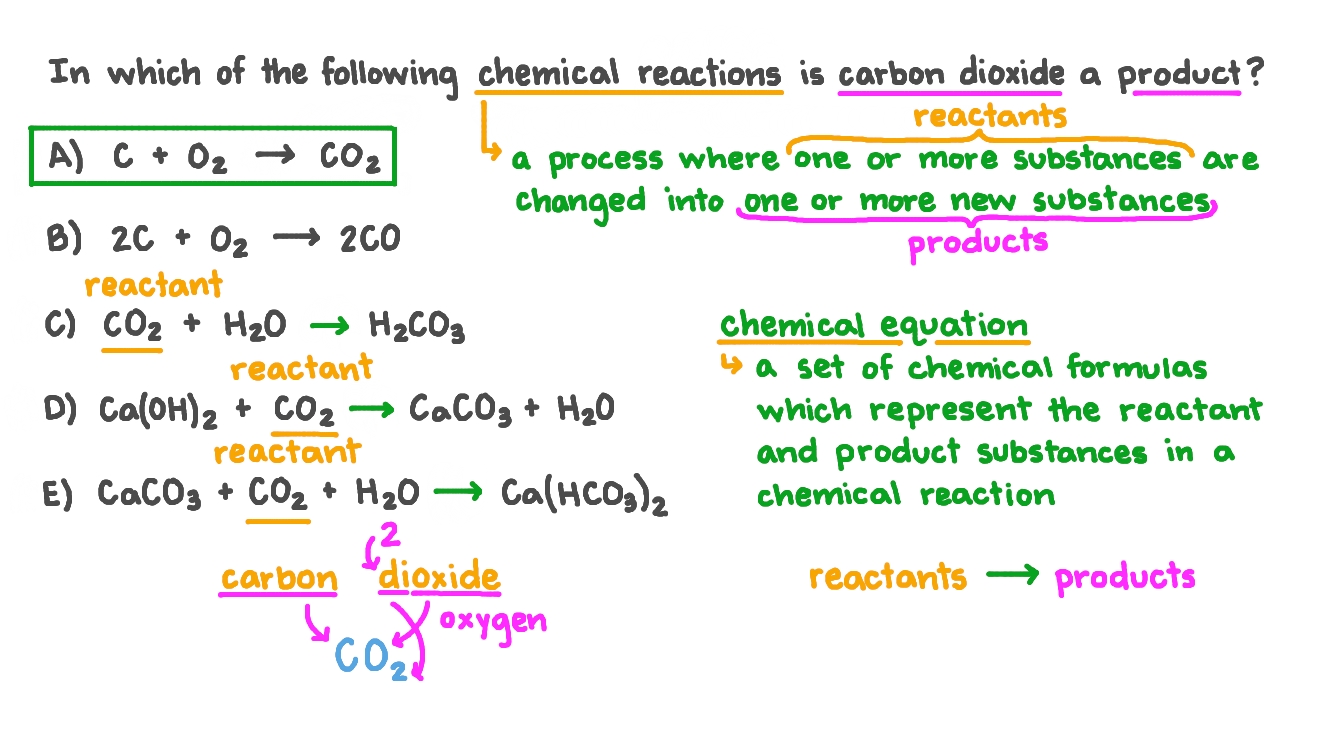
Question Video: Identifying the Chemical Formula of the Substance Produced When Carbon Dioxide Dissolves in Water | Nagwa
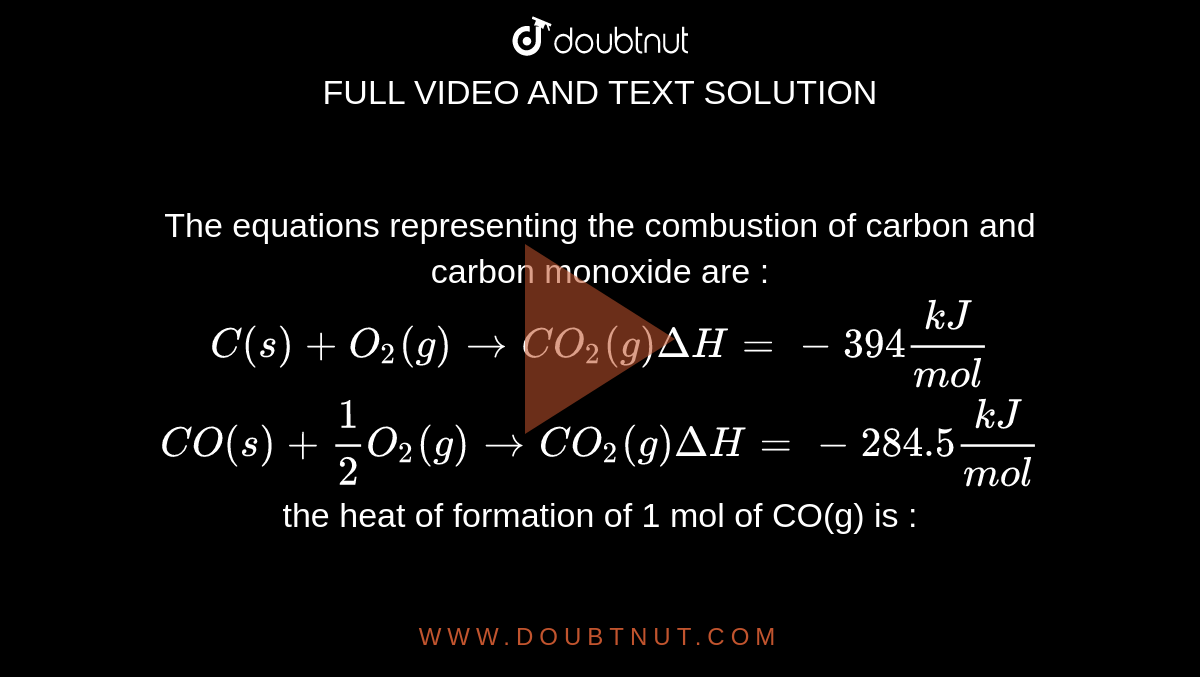
The enthalpies of combustion of carbon and carbon monoxide are -393.5 and –283kJ mol^–1 respectively. - Sarthaks eConnect | Largest Online Education Community
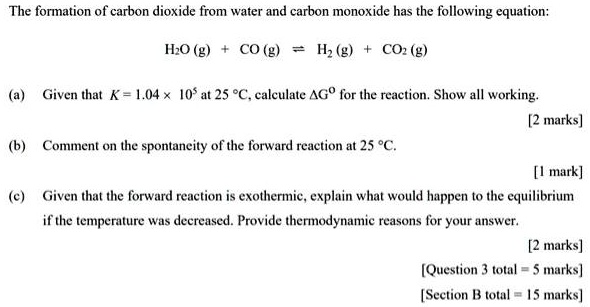
SOLVED: The formation of carbon dioxide from water and carbon monoxide has the following equation: IO (g) CO (g) Iz (g) COz (g) Given that K = 1.04 * 10' at 25 %