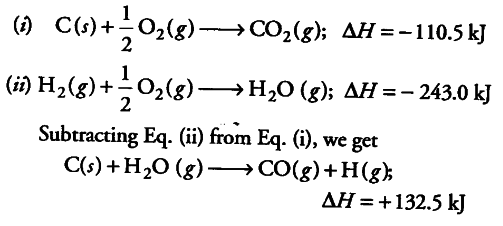![SOLVED: The rate law for the formation of carbon dioxide from carbon monoxide and oxygen is given by: 2CO(g) + 0,(g) 2C0,(g) Rate = k [CO][02] Which one of the following mechanism SOLVED: The rate law for the formation of carbon dioxide from carbon monoxide and oxygen is given by: 2CO(g) + 0,(g) 2C0,(g) Rate = k [CO][02] Which one of the following mechanism](https://cdn.numerade.com/ask_images/736b473b932d4d048614f30fae3d5333.jpg)
SOLVED: The rate law for the formation of carbon dioxide from carbon monoxide and oxygen is given by: 2CO(g) + 0,(g) 2C0,(g) Rate = k [CO][02] Which one of the following mechanism

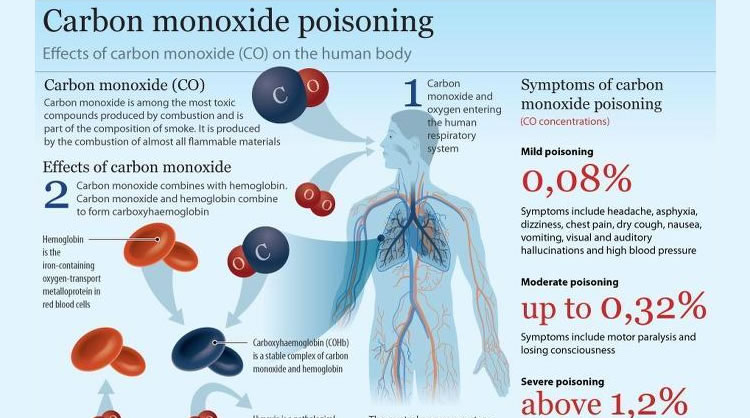
The heats of combustion of carbon and carbon monoxide are - 393.5 and - 283.5 kJ mol ^-1 , respectively. The heat of formation (in kJ) of carbon monoxide per mole is:
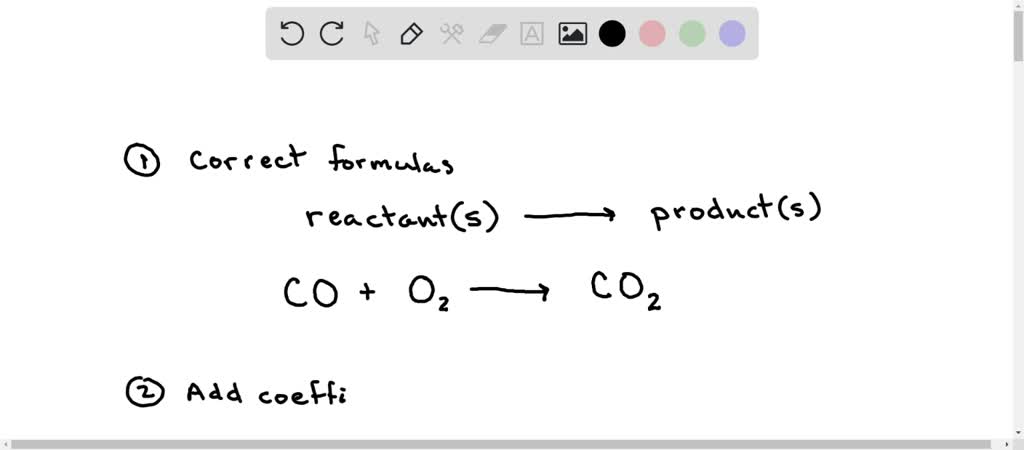
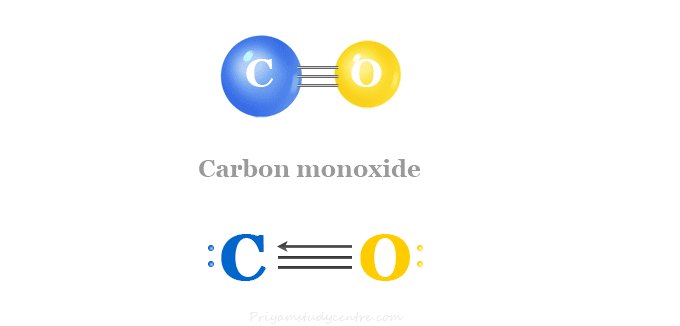
SOLVED: Write a balanced equation for the following reaction: When carbon monoxide combines with oxygen, carbon dioxide is formed. (Use the lowest possible coefficients. Omit states of matter.) Reactants Products + Step