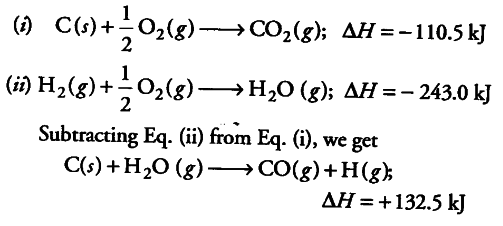The heat of formation of CO(g) and CO2(g) are Δ H = - 110 and Δ H = - 393kJmmol^-1 respectively. What is the heat of reaction (Δ H) (in kJ mol^-1 )

The heat of formation of CO(g) and CO2(g) are Δ H = - 110 and Δ H = - 393kJmmol^-1 respectively. What is the heat of reaction (Δ H) (in kJ mol^-1 )

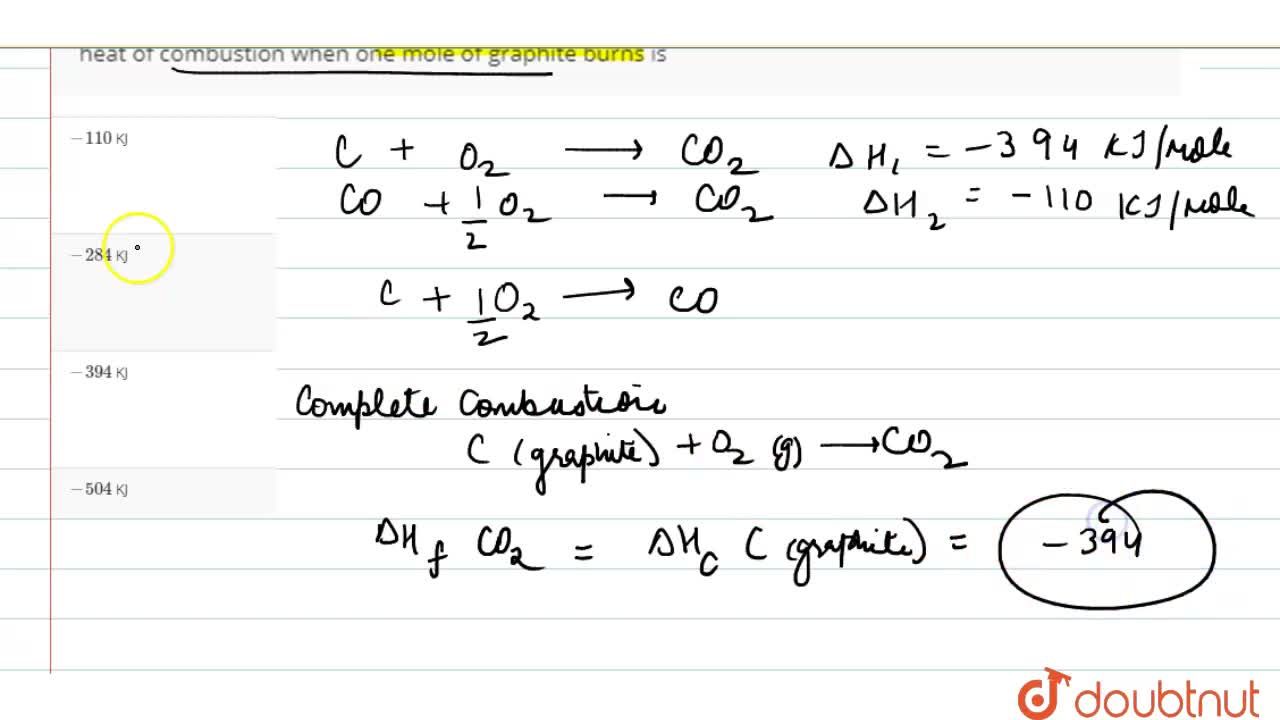
Given standard enthalpy of formation of CO ( - 110 KJ mol^-1 ) and C O2 ( - 394 KJ mol^-1 ). The heat of combustion when one mole of graphite burns is:
What is the enthalpy of formation of carbon monoxide in KJ/mol ? C(s) + O2(g) --> CO2(g) ΔH° = -393 kJ 2CO(g) + O2(g) --> 2CO(g) ΔH° = -588 kJ | Socratic

41524992Given standard enthalpy of formation of `CO(-110 \"KJ mol\"^(-1))` and `CO_(2)(-394 \"KJ mol - YouTube
Heat of combustion of carbon monoxide is 283.5 kJ/mole the heat released when 55g of carbon dioxide formed from carbon monoxide

The heat of formations of CO(g) and CO2 (g) are - 26.4 kcal and - 94.0 kcal receptively, The heat of combination of carbon monoxide will be

Calculate the enthalpy of formation of carbon monoxide (CO) from the following data: (i) C (s) + O2 (g) → - Brainly.in

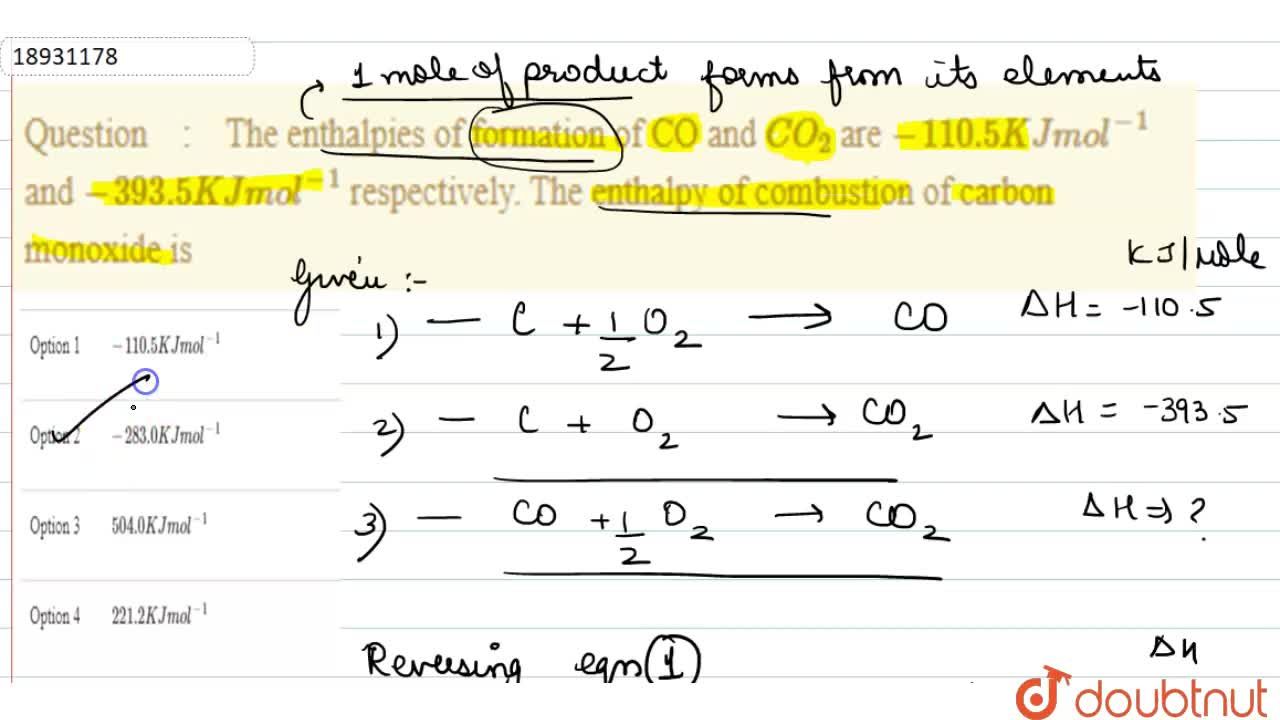
The enthalpies of formation of CO and `CO_(2)` are `-110.5 KJ mol^(-1)` and `-393.5 KJ mol^(-1)` - YouTube