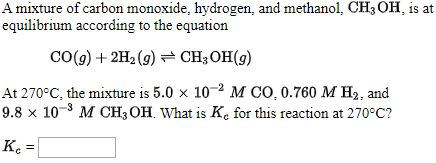Acceptorless Dehydrogenation of Methanol to Carbon Monoxide and Hydrogen using Molecular Catalysts - Kaithal - 2021 - Angewandte Chemie International Edition - Wiley Online Library

Methanol production via direct carbon dioxide hydrogenation using hydrogen from photocatalytic water splitting: Process development and techno-economic analysis - ScienceDirect
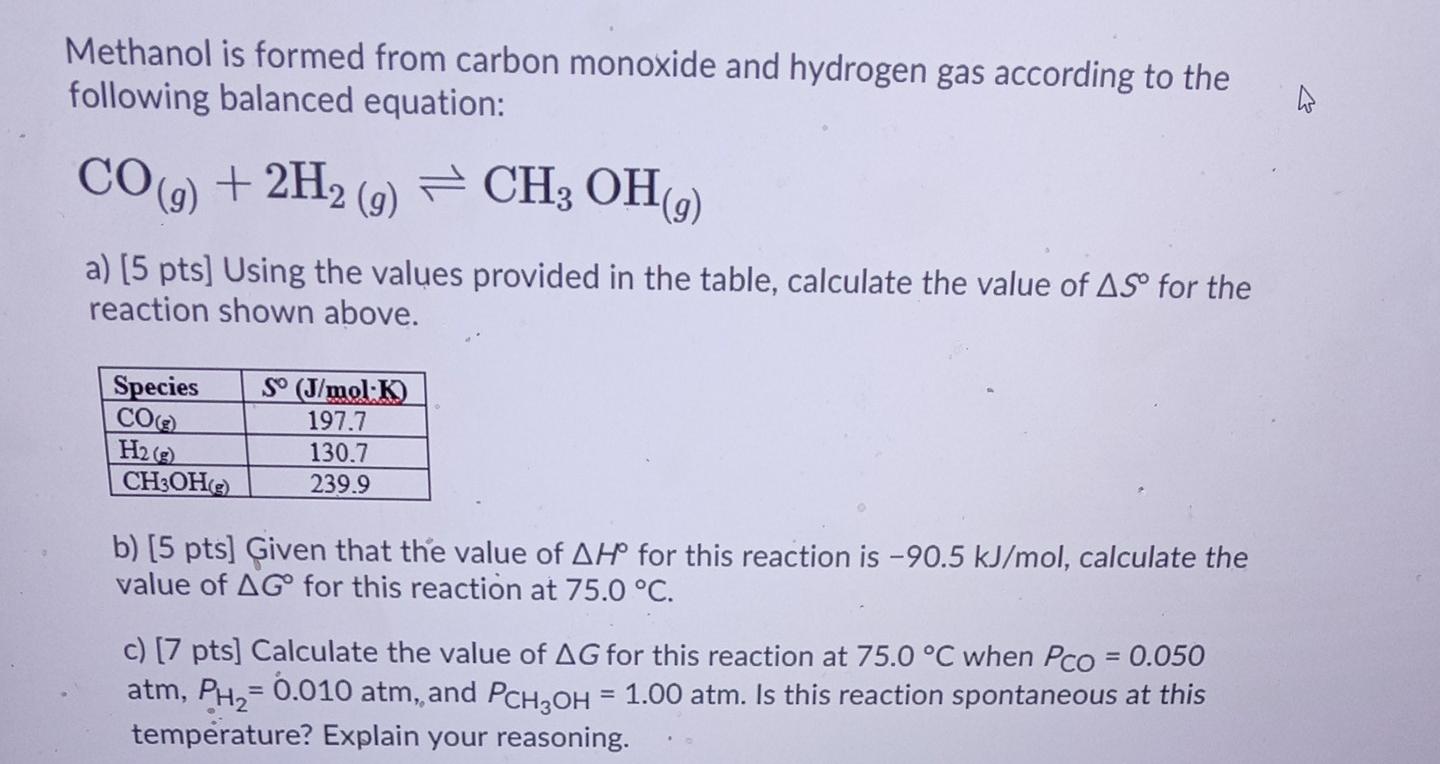
![SOLVED: Methanol is formed from carbon monoxide and hydrogen gas according to the following balanced equation: CO(g) 2Hz (9) CHs ' OH(g) a) [5 pts] Using the values provided in the table; SOLVED: Methanol is formed from carbon monoxide and hydrogen gas according to the following balanced equation: CO(g) 2Hz (9) CHs ' OH(g) a) [5 pts] Using the values provided in the table;](https://cdn.numerade.com/ask_images/16c04d240456462d8b6ebcc0b2d26908.jpg)
SOLVED: Methanol is formed from carbon monoxide and hydrogen gas according to the following balanced equation: CO(g) 2Hz (9) CHs ' OH(g) a) [5 pts] Using the values provided in the table;

carbon monoxide reacts with hydrogen under certain condition to form methanol (CH3OH). write the balanced - Brainly.in

Power-law kinetics of methanol synthesis from carbon dioxide and hydrogen on copper–zinc oxide catalysts with alumina or zirconia supports - ScienceDirect

hydrocarbons - Why carbon monoxide reacts with hydrogen to give different products in the presence of different catalysts - Chemistry Stack Exchange
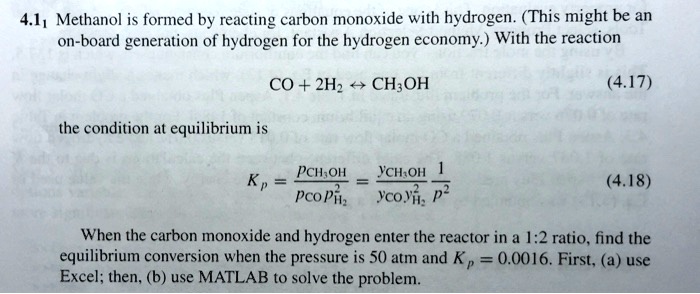
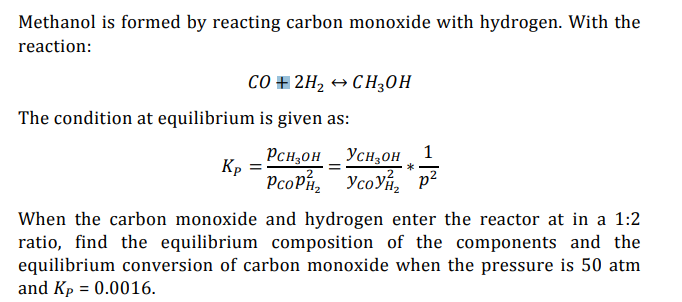
SOLVED: 41 Methanol is formed by reacting carbon monoxide with hydrogen. (This might be an on-board generation of hydrogen for the hydrogen economy) With the reaction CO + 2Hz CH;OH (4.17) the

Alcohol-Assisted Hydrogenation of Carbon Monoxide to Methanol Using Molecular Manganese Catalysts | JACS Au

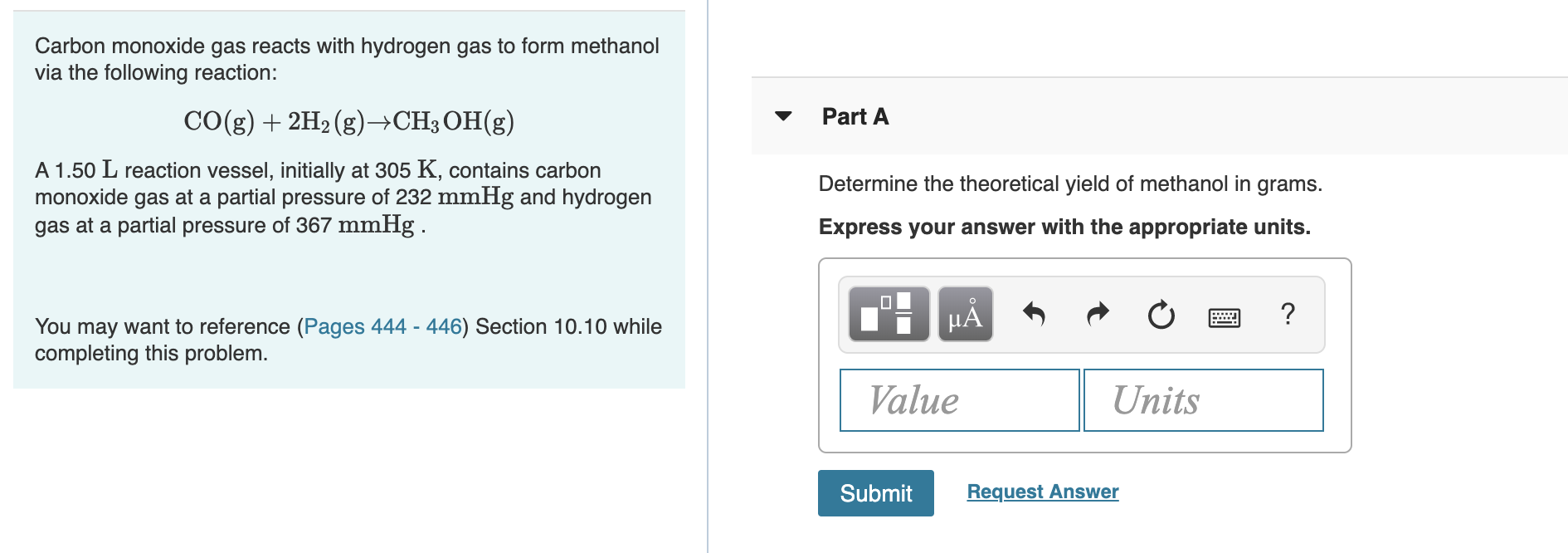
Methanol can be prepared synthetically by heating carbon monoxide and hydrogen gases under pressure in the presence of a catalyst. The reaction is CO(g) + 2H2(g)→CH3)H(l) Determine the enthalpy of this reaction

Carbon monoxide reacts with hydrogen under certain conditions to from methanol `(CH_(3)OH)` - YouTube