hydrocarbons - Why carbon monoxide reacts with hydrogen to give different products in the presence of different catalysts - Chemistry Stack Exchange

Effect of reaction pressure on equilibrium a hydrogen, b methane, c... | Download Scientific Diagram
![SOLVED: Methanol is formed from carbon monoxide and hyarogen gas according to the following balanced equation: CO(g) 2Hz (g) CH; OHcg) a) [5 pts] Using the values provided in the table; calculate SOLVED: Methanol is formed from carbon monoxide and hyarogen gas according to the following balanced equation: CO(g) 2Hz (g) CH; OHcg) a) [5 pts] Using the values provided in the table; calculate](https://cdn.numerade.com/ask_images/02c7b6f2431a40e6bdae48e298e6cc4a.jpg)
SOLVED: Methanol is formed from carbon monoxide and hyarogen gas according to the following balanced equation: CO(g) 2Hz (g) CH; OHcg) a) [5 pts] Using the values provided in the table; calculate

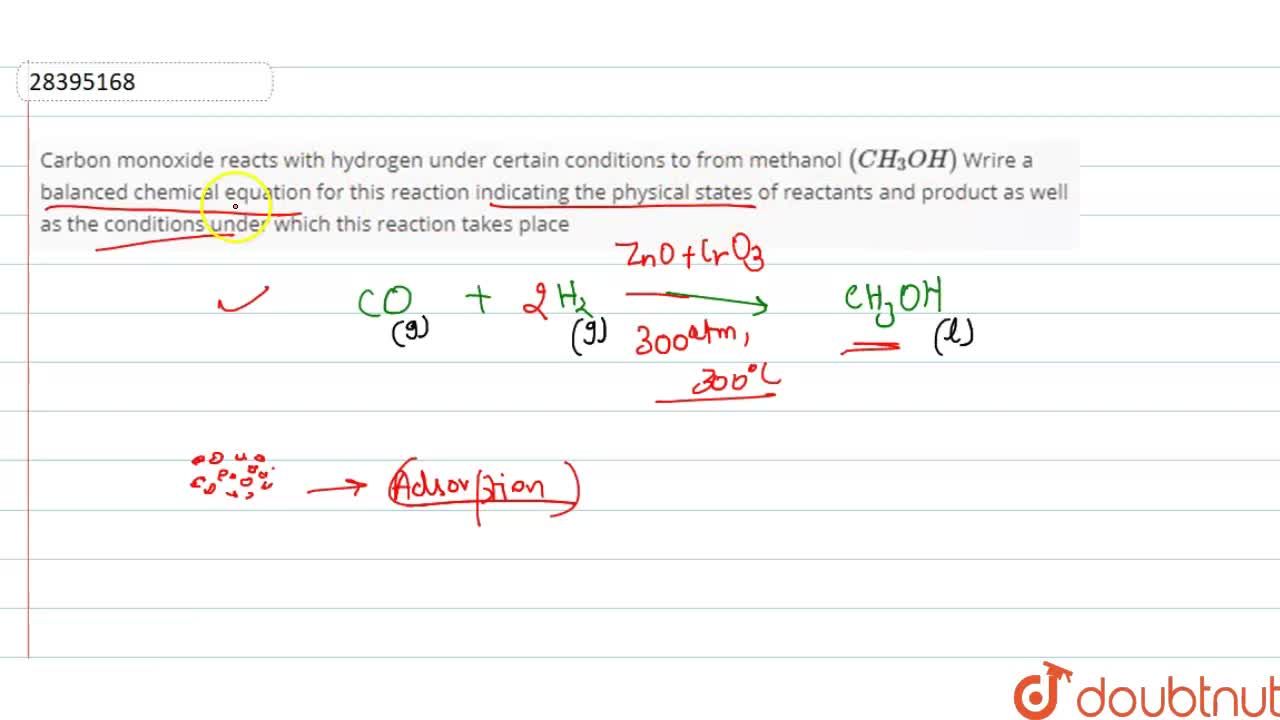
carbon monoxide reacts with hydrogen under certain condition to form methanol (CH3OH). write the balanced - Brainly.in

Hydrogen, oxygen and carbon dioxide are taken in containers of 2 l volume each. Compare the number of molecules of the three gases under same conditions of temperature and pressure.
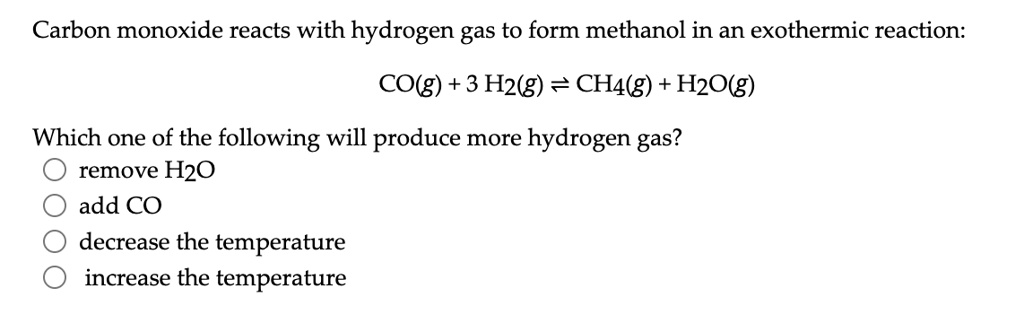
SOLVED: Carbon monoxide reacts with hydrogen gas to form methanol in an exothermic reaction: CO(g) 3 Hz(g) = CH4(g) + H2Okg) Which one of the following will produce more hydrogen gas? remove

Propene reacts with carbon monoxide and hydrogen in presence of cobalt carbonyl catalyst at high - YouTube

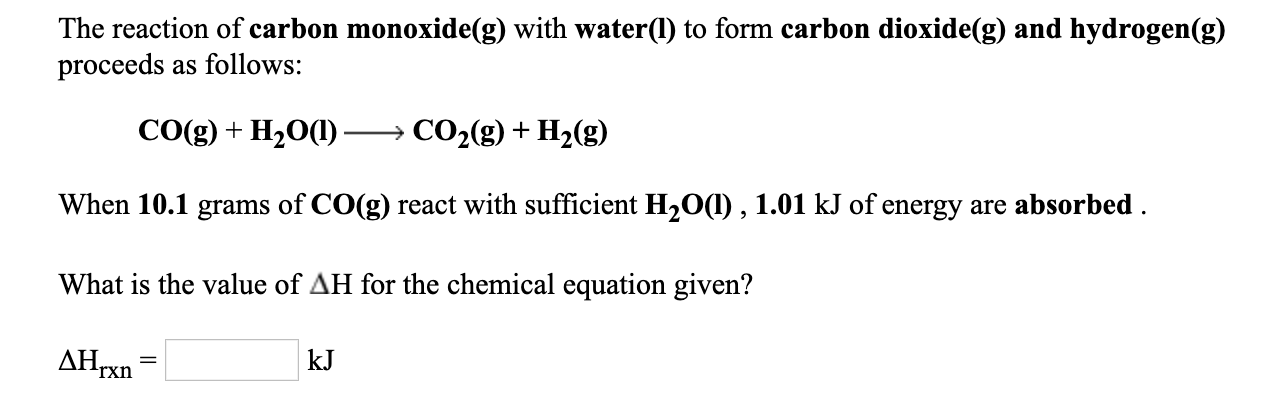
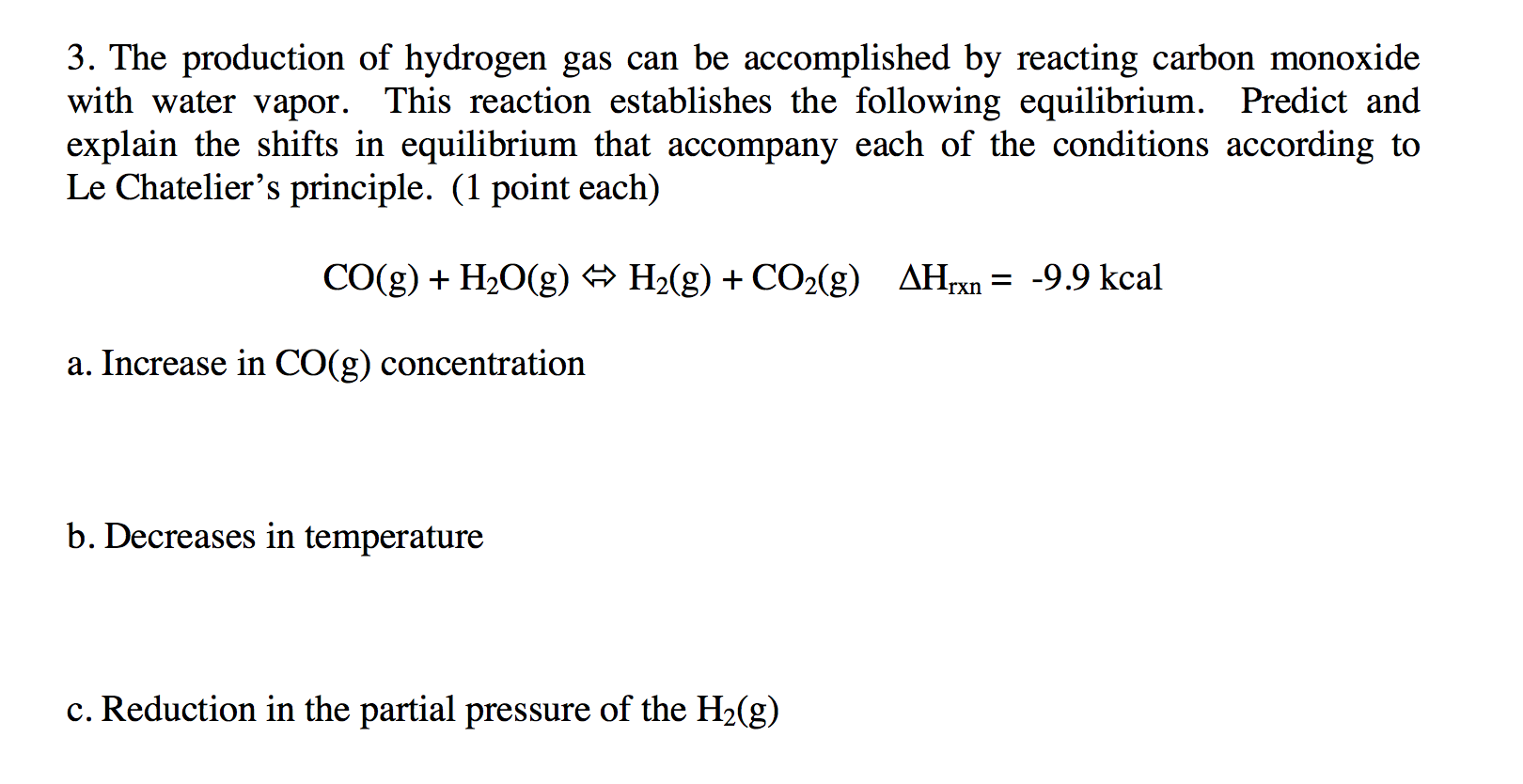
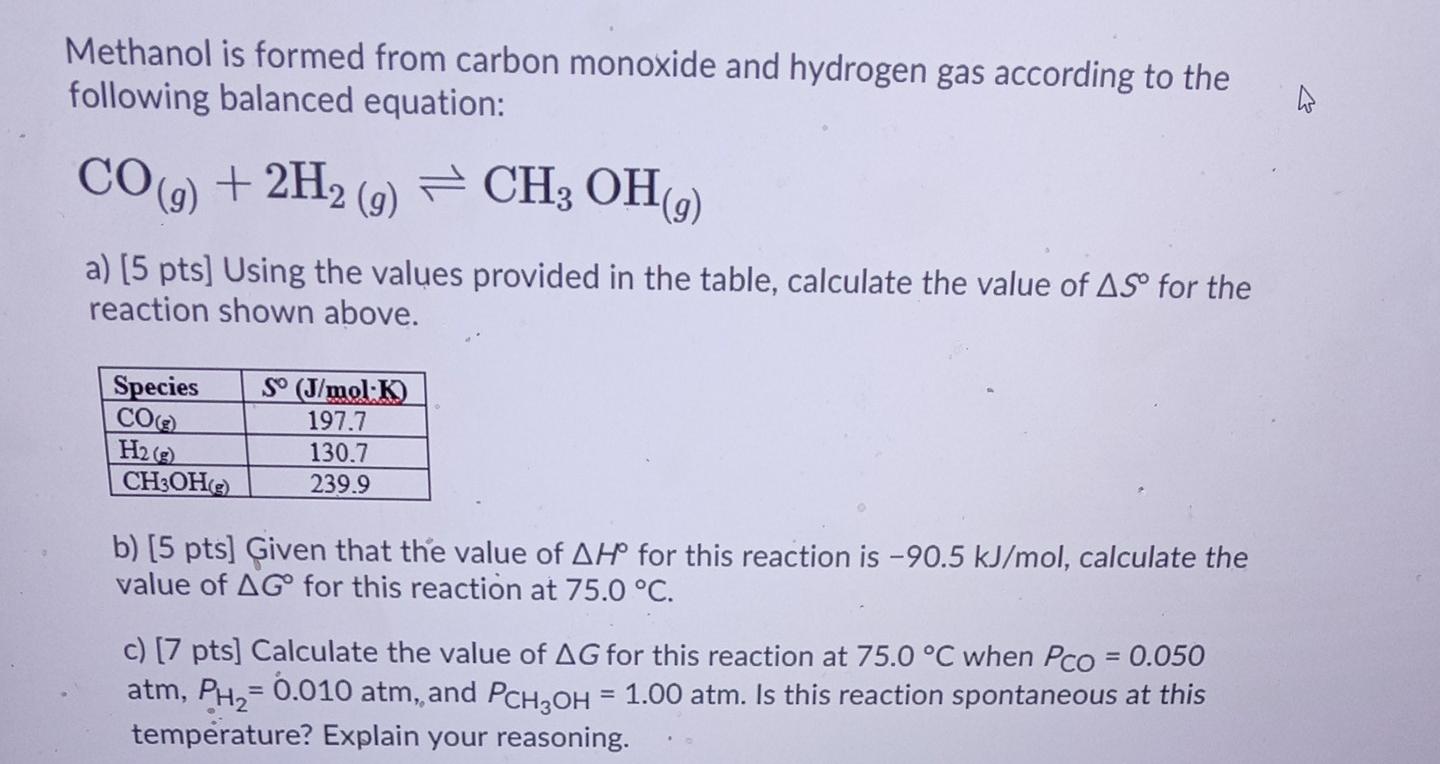






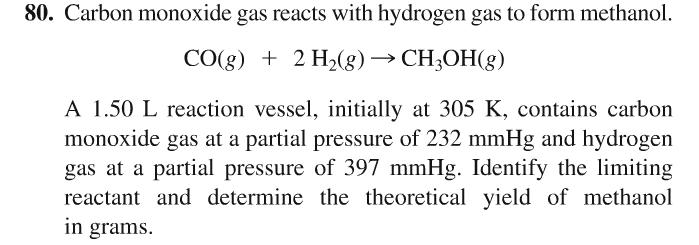

.jpg)