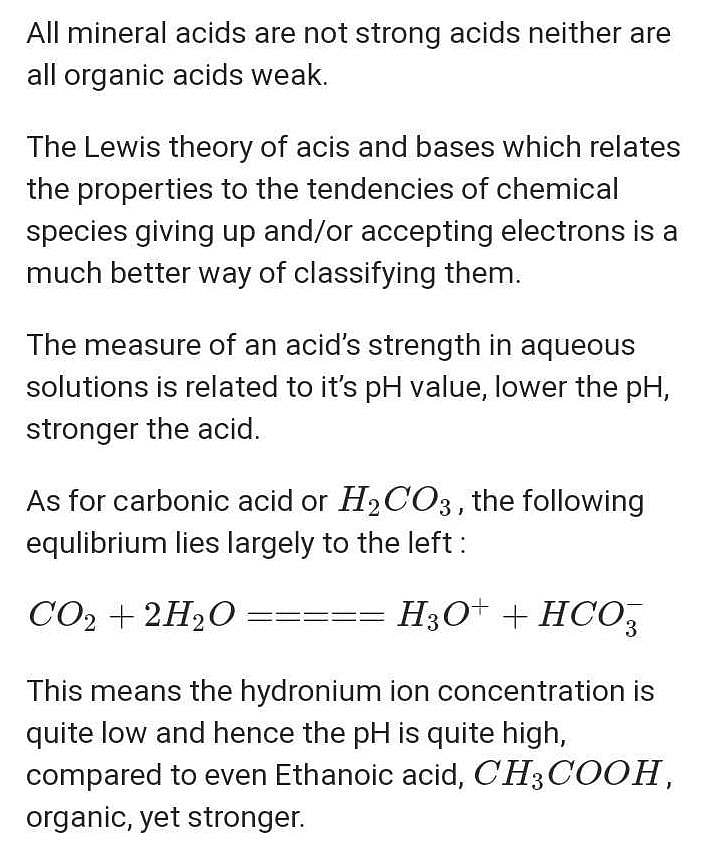
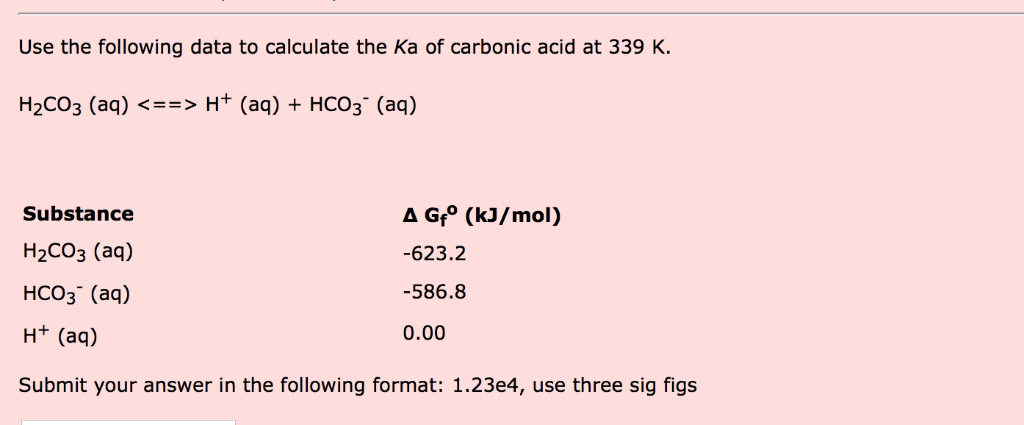
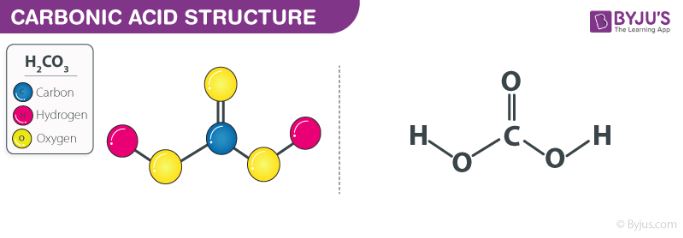
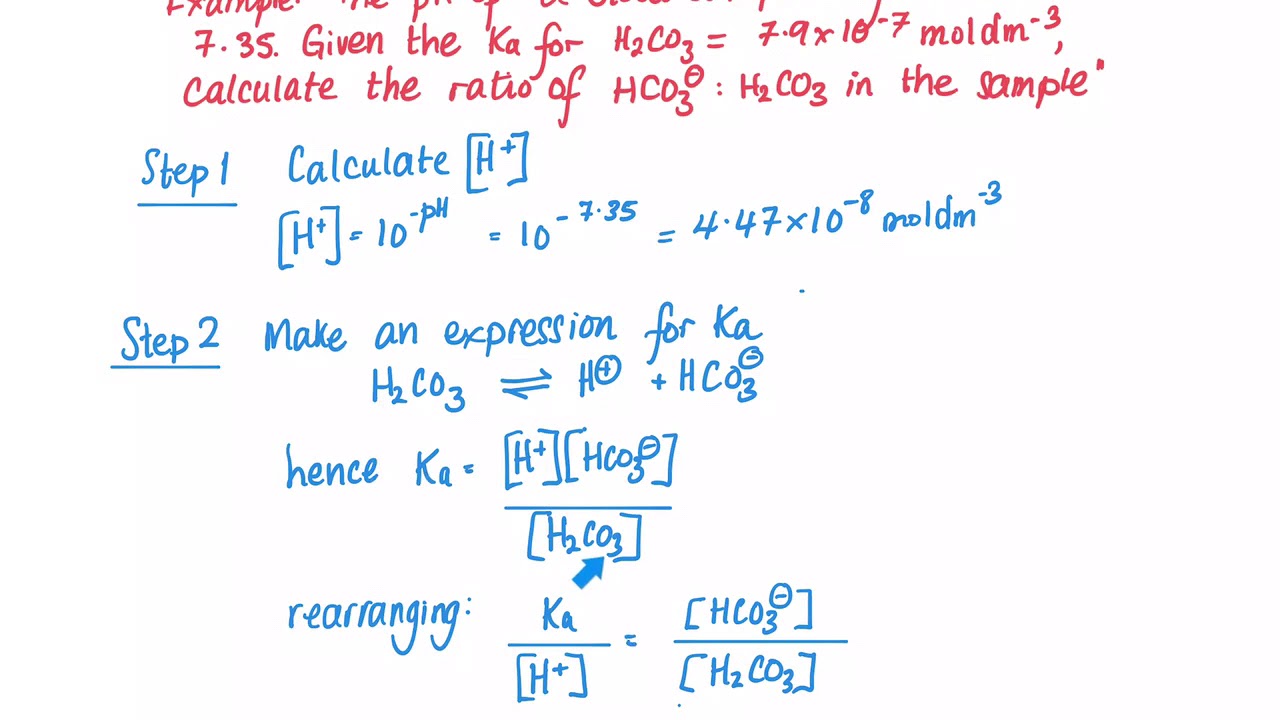
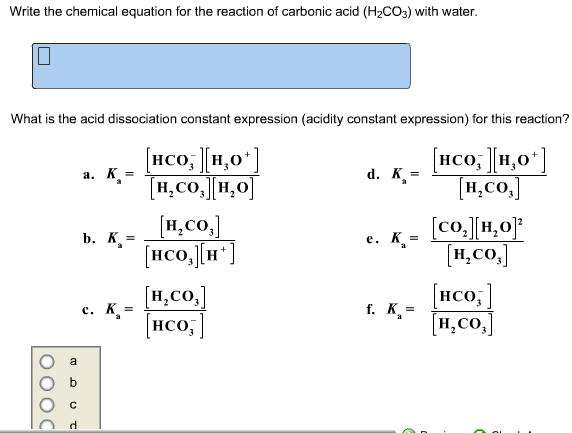
Why is carbonic acid a weak acid even though it gets completely dissociated into H+ and CO3- ions? - Quora

pH calculations and more in fundamentals of pharmaceutics. : Calculate pH and buffer capacity of a carbonic acid/ bicarbonate buffer solution.
![SOLVED: Show that [H KInRIn: From the carbonic acid dissociation equilibria, show that Ki[CO2(aq)] [HCOz ] [H+1 KiKz[COz(aq)] [CO 1 [H+12 Write the charge balance for the solution in the sensor compart- SOLVED: Show that [H KInRIn: From the carbonic acid dissociation equilibria, show that Ki[CO2(aq)] [HCOz ] [H+1 KiKz[COz(aq)] [CO 1 [H+12 Write the charge balance for the solution in the sensor compart-](https://cdn.numerade.com/ask_images/817847570bbc42b6a424230ff309e013.jpg)
SOLVED: Show that [H KInRIn: From the carbonic acid dissociation equilibria, show that Ki[CO2(aq)] [HCOz ] [H+1 KiKz[COz(aq)] [CO 1 [H+12 Write the charge balance for the solution in the sensor compart-

The Ka of carbonic acid is 4.3 x 10-7. H2CO3 = H+ + HCO3 This means that H2co3 is a____. A.good - Brainly.com

SOLVED: acid chemical formula Ka acetic butyric carbonic formic hypochlorous monohydrogen carbonate nitrous propionic HCzH3Oz HCAH-Oz HzCOs HCHO HCIO HCOz 1.8 x 10 15*10- 43x 10-7 1.8 x 10-4 3.0 x 10-8

Calculate the pH (nearest integer) of 0.010M NaHCO3 solution. K1 = 4.5 × 10^-7 and K2 = 4.7 × 10^-11 for carbonic acid.