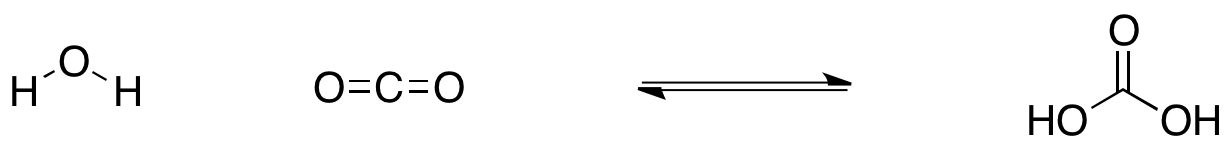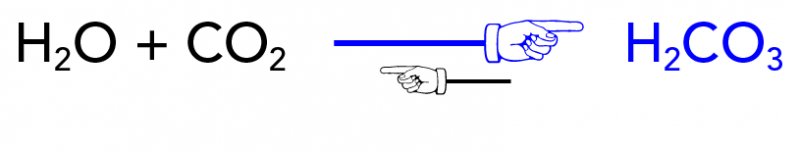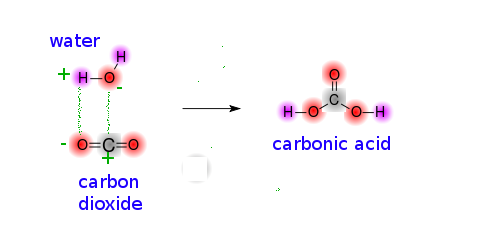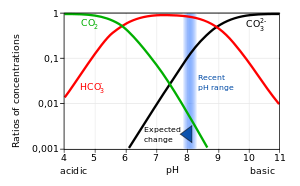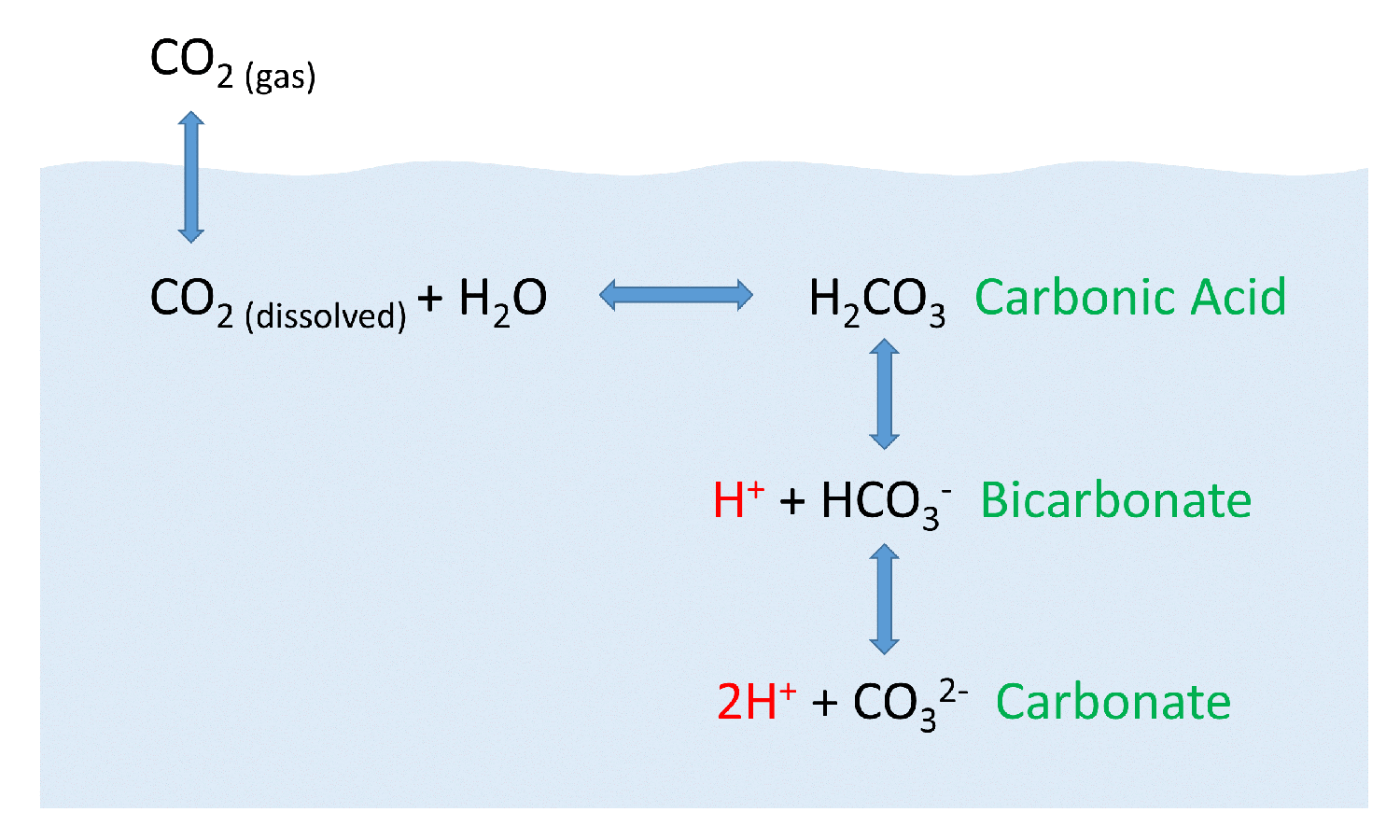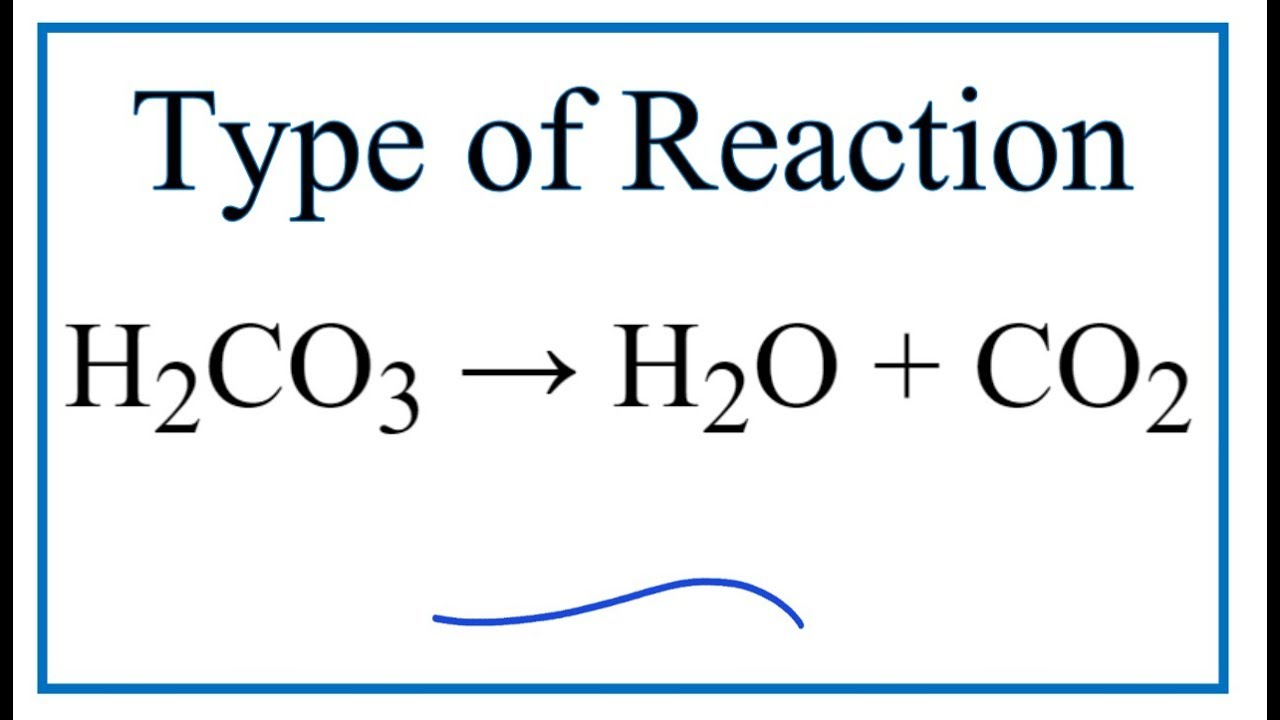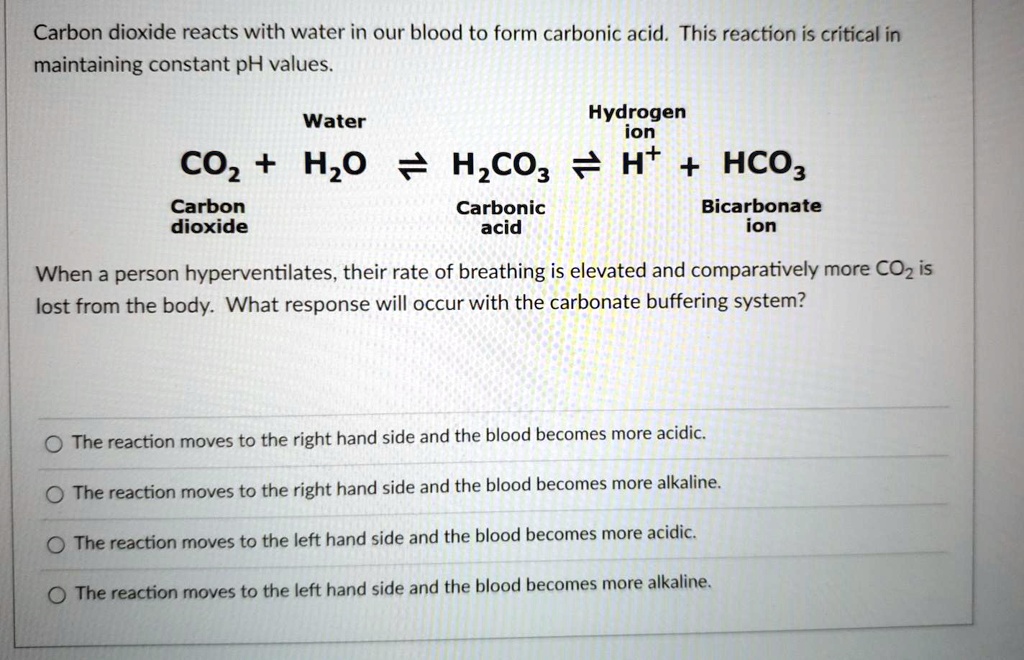
SOLVED: Carbon dioxide reacts with water in our blood to form carbonic acid. This reaction is critical in maintaining constant pH values Water Hydrogen ion HzCOz H+ HCO3 Carbonic Bicarbonate acid ion
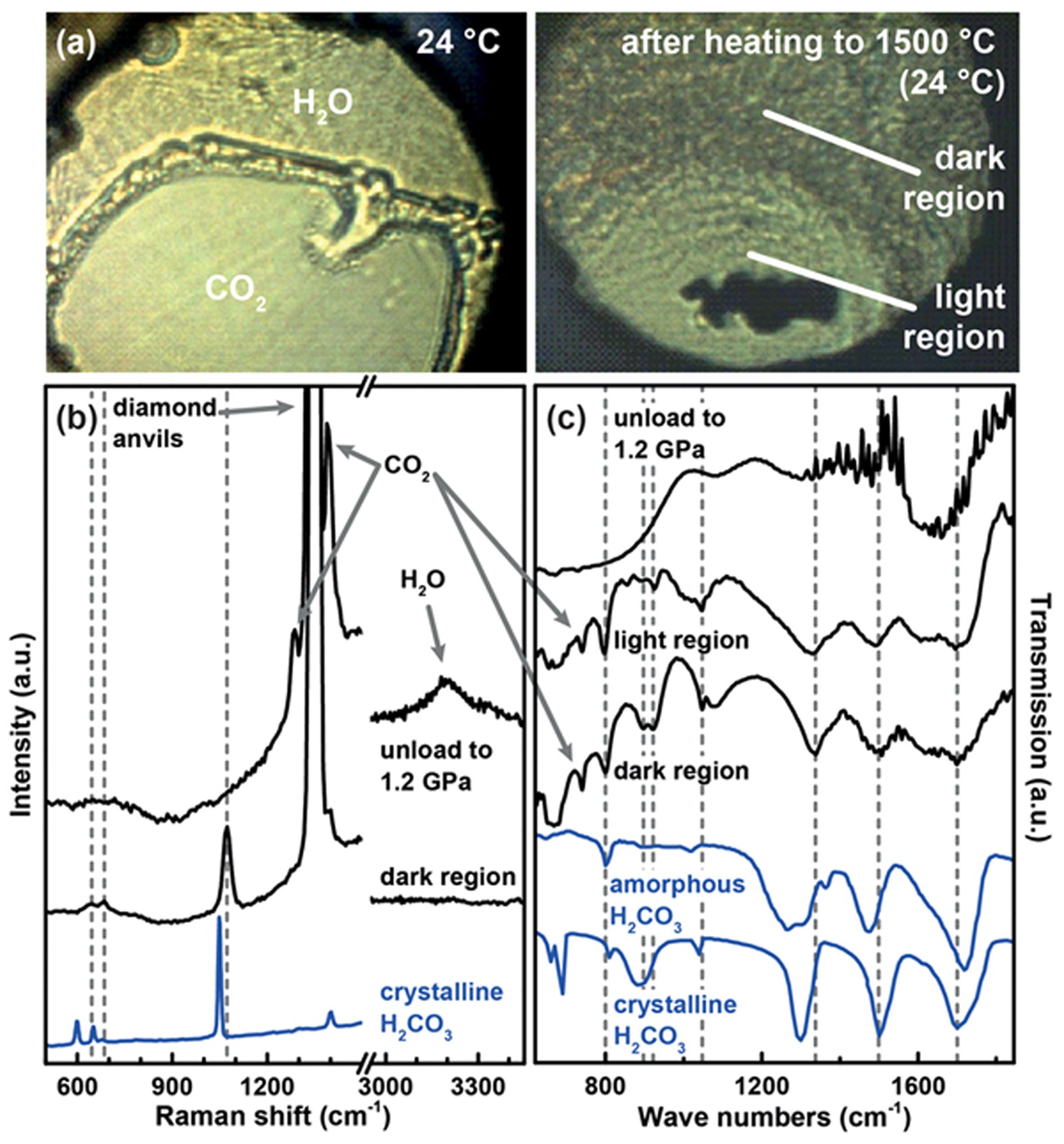
Stable solid and aqueous H2CO3 from CO2 and H2O at high pressure and high temperature | Scientific Reports

What is the Difference Between Carbonic Acid and Bicarbonate | Compare the Difference Between Similar Terms

Carbonic acid formation in the atmosphere. Processing of mineral dust... | Download Scientific Diagram