How is the combustion of carbon a redox reaction? I get the oxidation part, but where is the reduction? - Quora

Enthalpy of combustion of carbon to CO2 is -393.5KJ mol-1. Calculate the heat released upon..... - YouTube
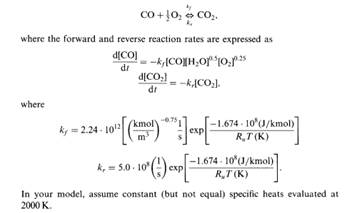
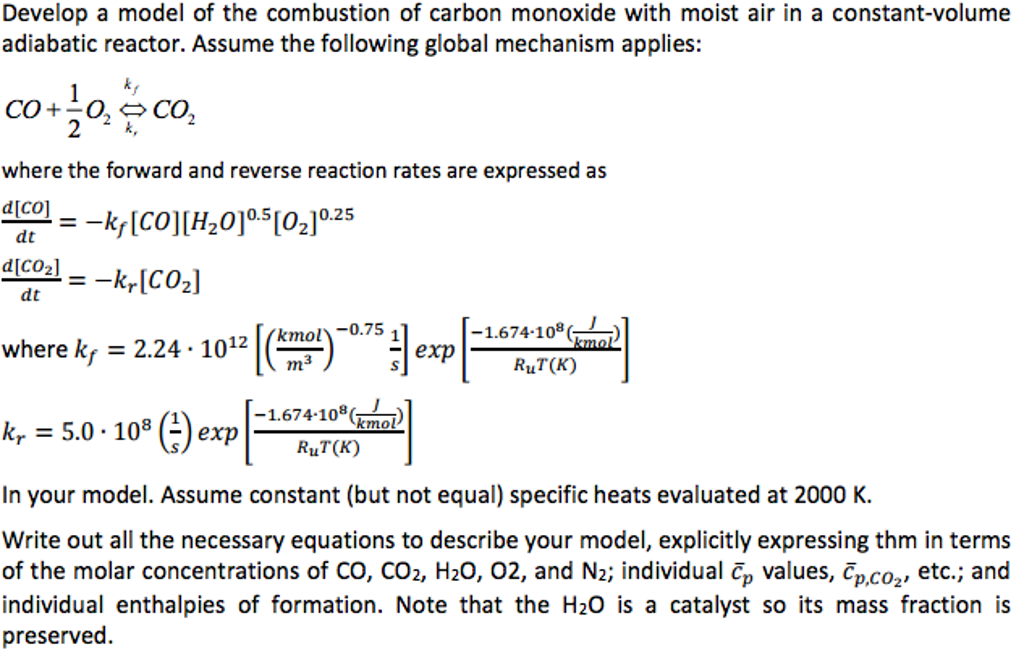
Solved) - Develop a model of the combustion of carbon monoxide with moist... - (3 Answers) | Transtutors
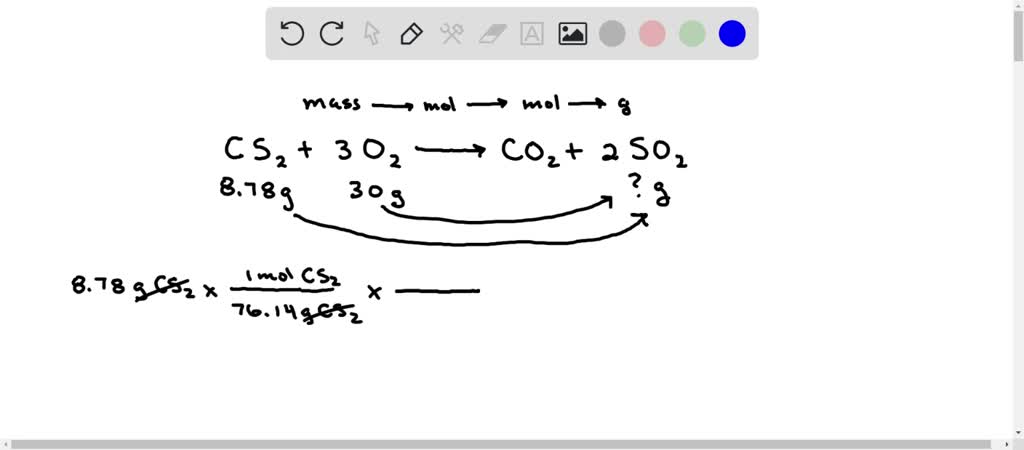
SOLVED: The combustion of carbon disulfide in the presence of excess oxygen yields carbon dioxide and sulfur dioxide according to the following UNBALANCED reaction: CS2 (g) + 3O2 (g) → CO2 (g) +

Enthalpy of combustion of carbon to CO2 is - 393.5 KJ/mole. The heat released upon the formation of 35.2g of CO2 from carbon and dioxygen gas is.
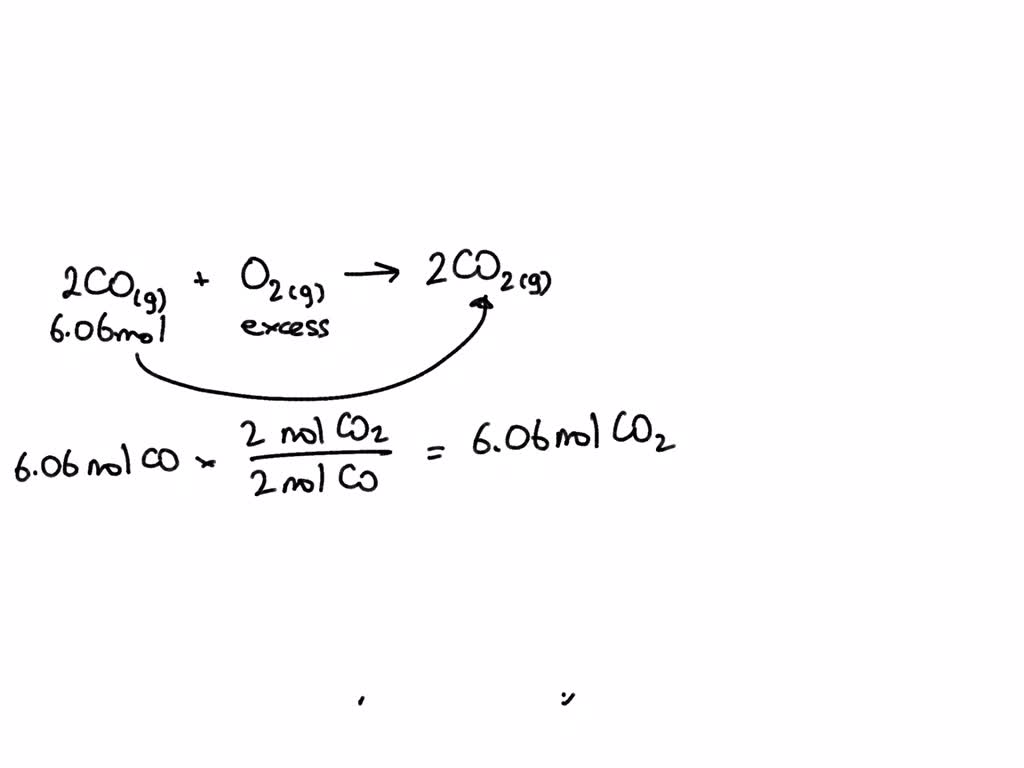
SOLVED: Consider the combustion of carbon monoxide (CO) in oxygen gas: 2CO(g) + O2(g) → 2CO2(g) Calculate the number of moles of CO2 produced if 6.06 moles of CO are reacted with

OneClass: The combustion of carbon monoxide is represented by the equation above Determine the value ...

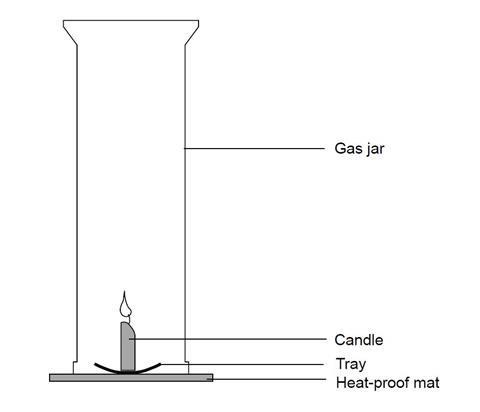
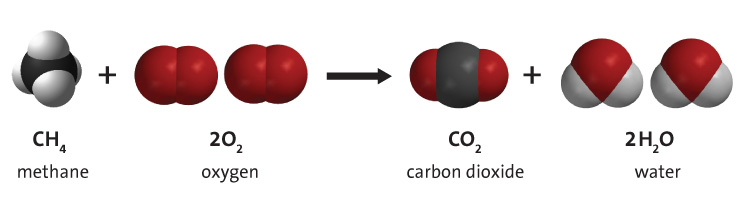
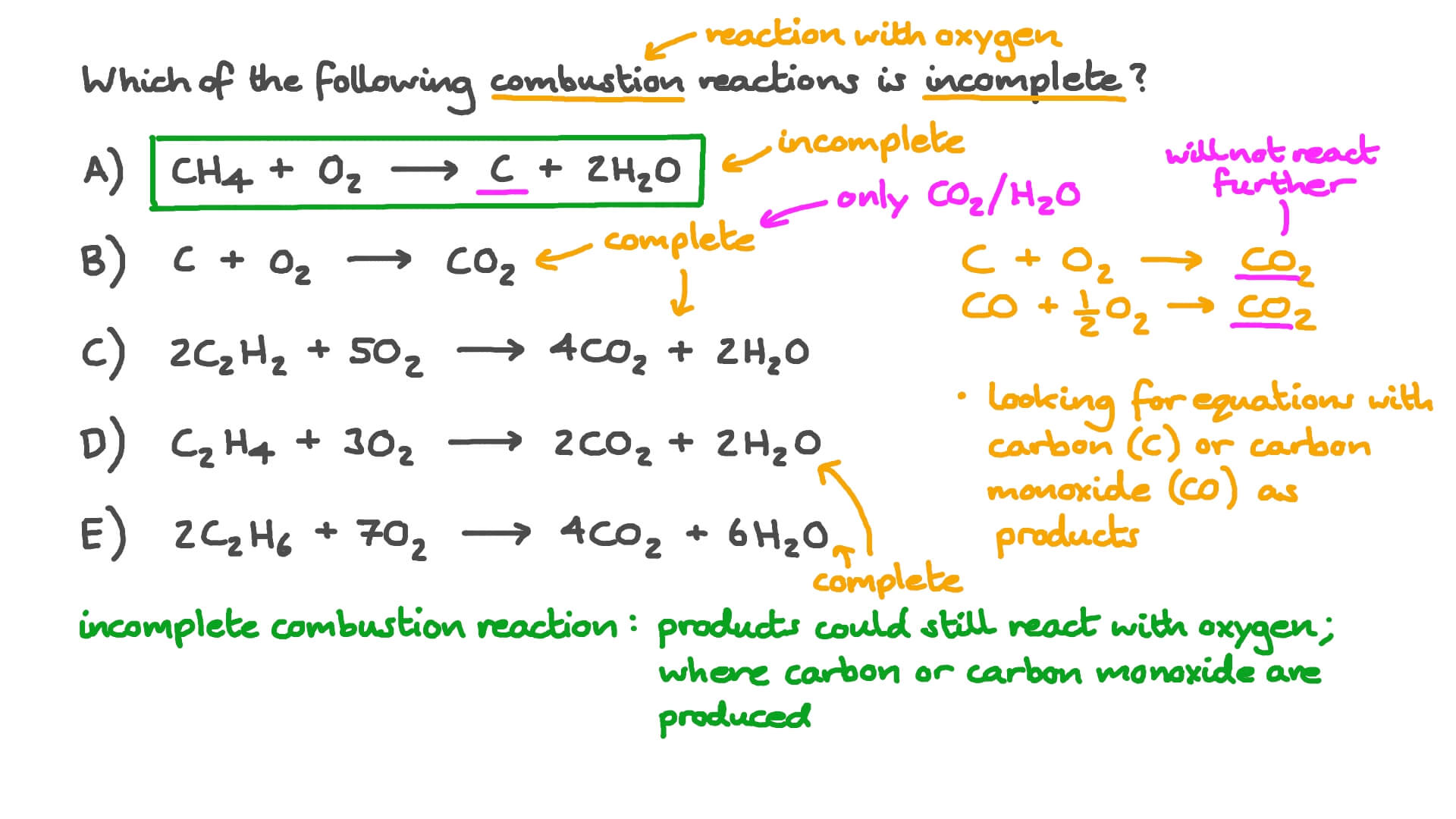
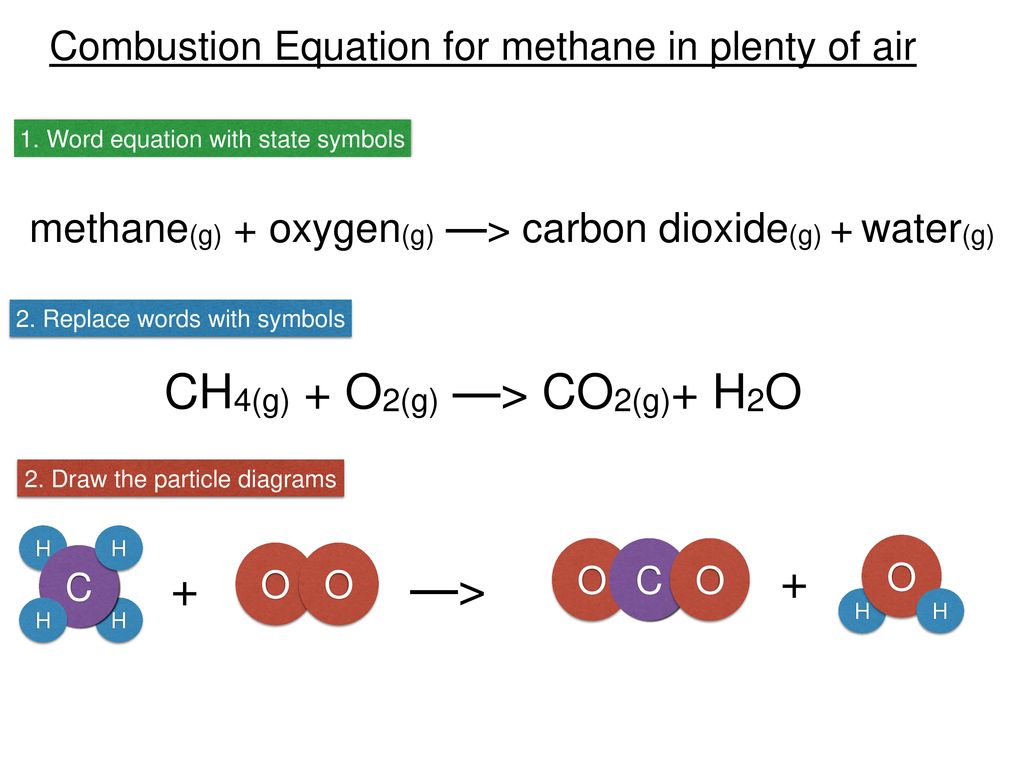
Combustion of hydrocarbons When hydrocarbons are heated in air they react with oxygen forming carbon dioxide or carbon monoxide or carbon, and water. The. - ppt download
62.The Enthalpies of combustion of carbon and carbon monoxide are 390 kJ and 278kJ respectively. The enthalpy of formation of carbon monooxide is? a) 669 kJ b) 112 kJ c) 112 kJ d) 668 kJ
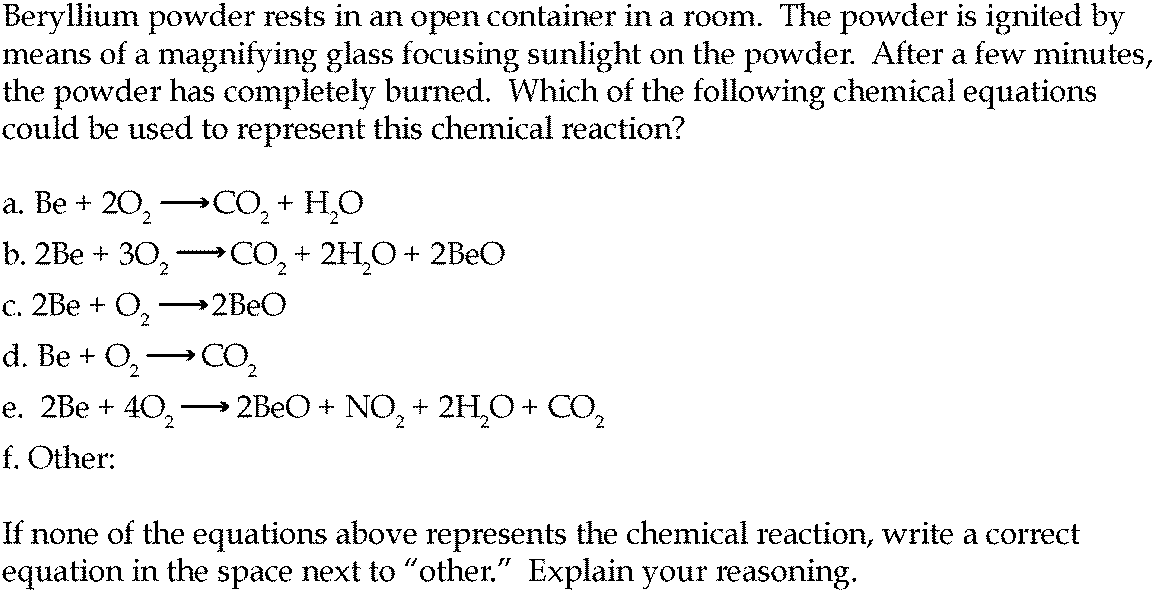
Combustion always produces carbon dioxide and water”: a discussion of university chemistry students' use of rules in place of principles - Chemistry Education Research and Practice (RSC Publishing) DOI:10.1039/C4RP00089G
20. One mole of carbonundergoes incomplete combustion to produce carbon monoxide. Calculate(AH Δ ) for the formation of CO at 298 KGiven R 8314 JK 1 mol 1

The enthalpies of combustion of carbon and carbon monoxide are `-390 kJ mol^(-1)` and `-278 kJ mo - YouTube