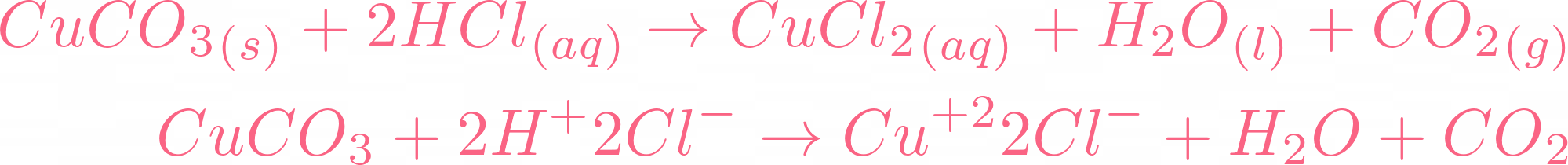Copper Carbonate,Senior Chemistry - Extended Experimental In-Industry News-Nickel Acetate,Cobalt Sulfate-Fairsky Industrial Co., Limited
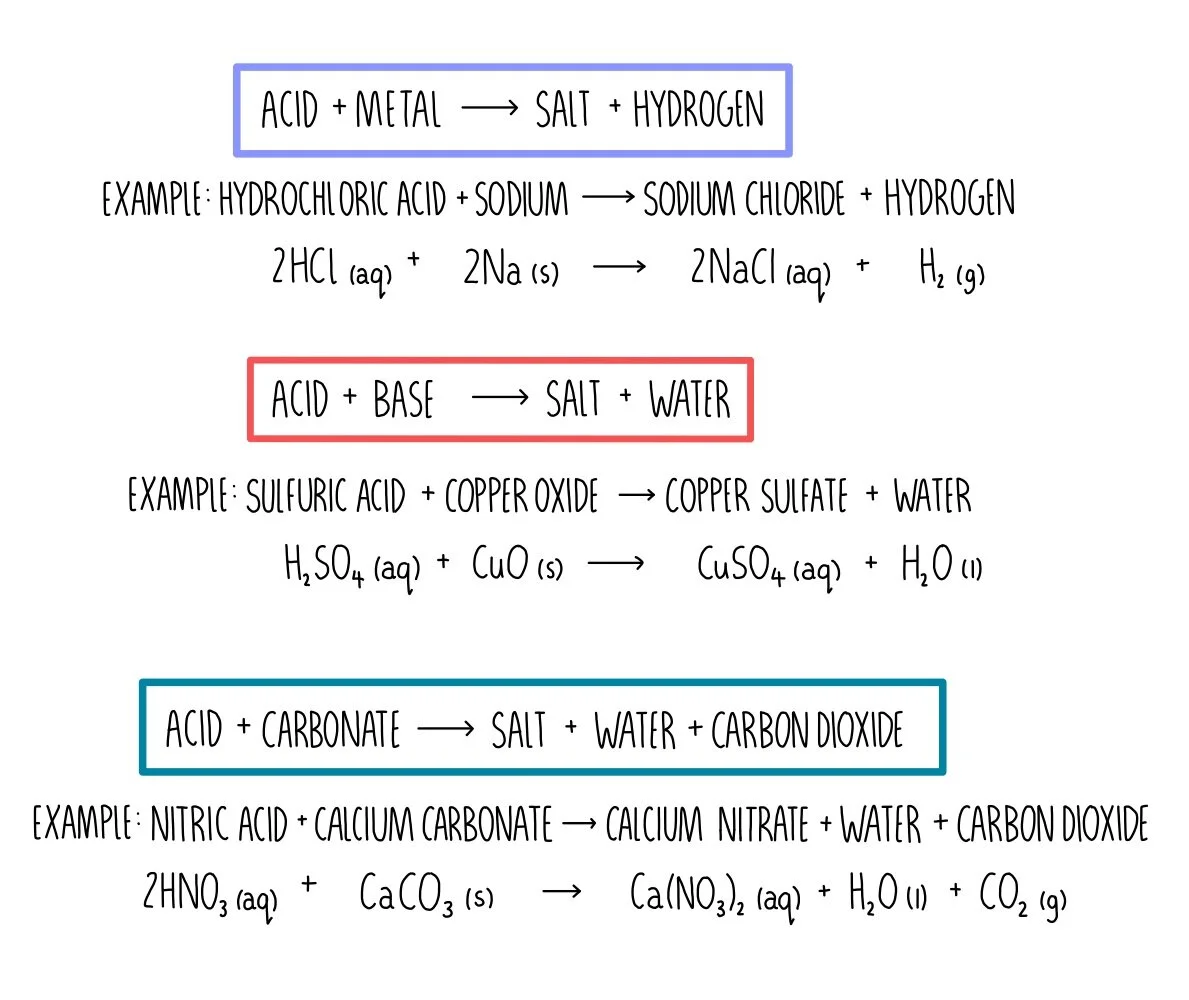
Dilute hydrochloric acid is added to copper carbonate. - Sarthaks eConnect | Largest Online Education Community
![Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition](https://icandochemistry942105908.files.wordpress.com/2021/05/mq2-3.jpg?w=640)
Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition

Q3 Give reasons why the following are considered as chemical changes 1 Copper Carbonate on heat give...

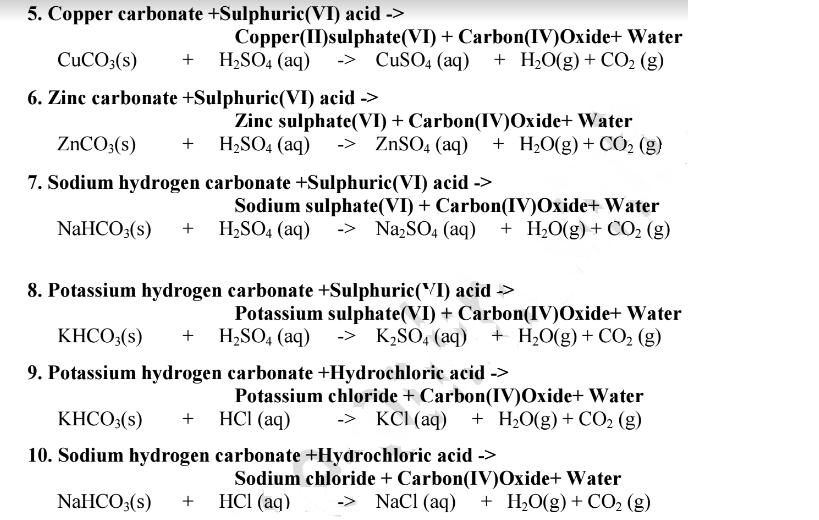
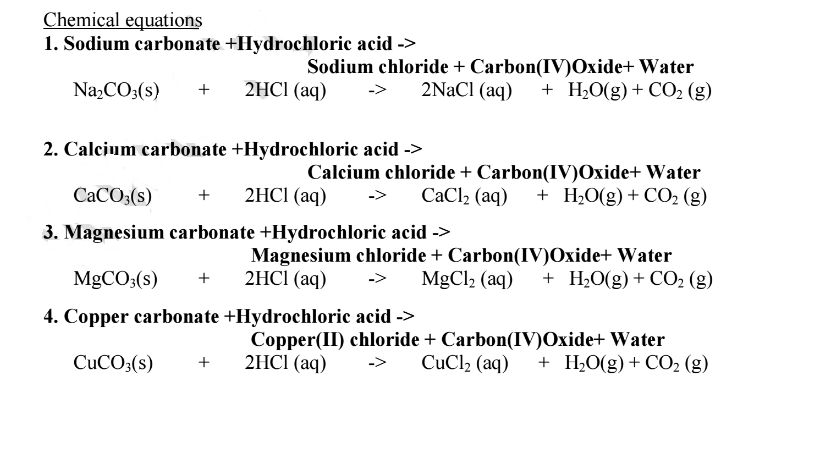
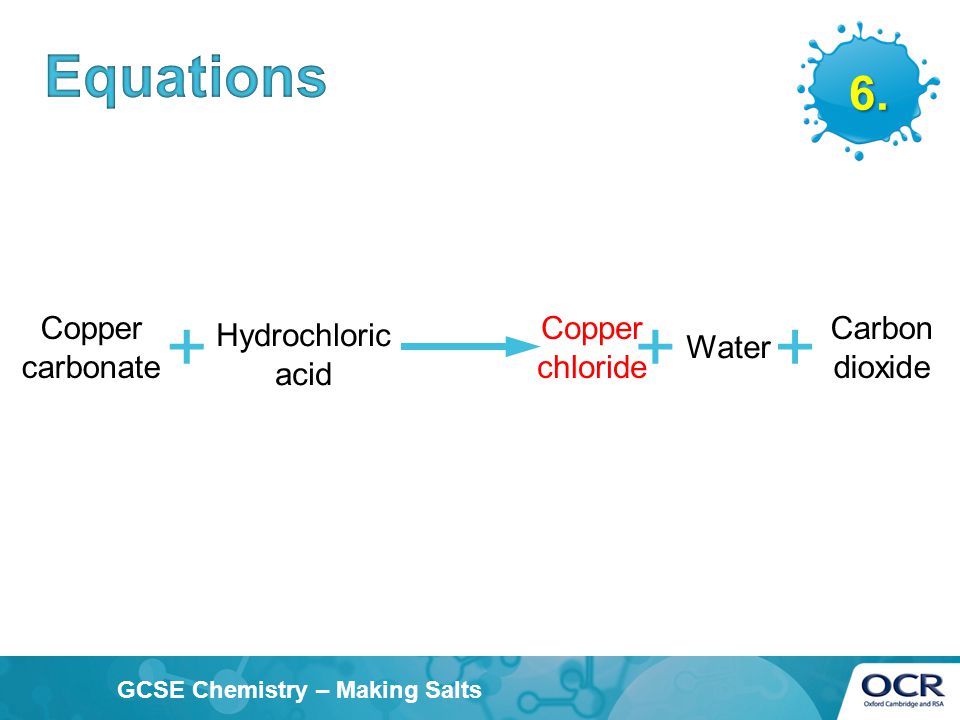
SOLVED: Copper carbonate (CuCO3) reacts with hydrochloric acid (HCl) according to this equation: CuCO3(s) + 2HCl(aq) → CuCl2(aq) + H2O(l) + CO2(g). Which statement correctly describes the substances in this reaction? A.
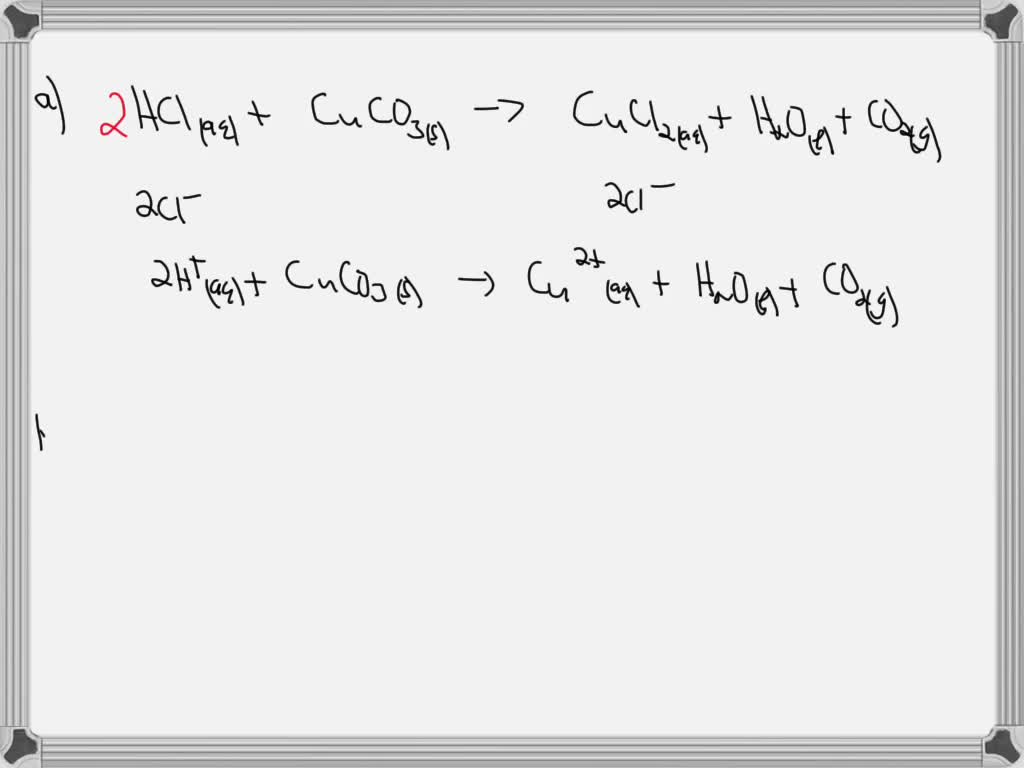
SOLVED: A. Write a net ionic equation for the reaction that occurs when excess hydrochloric acid (aq) and copper(II) carbonate are combined. B. Write a net ionic equation for the reaction that