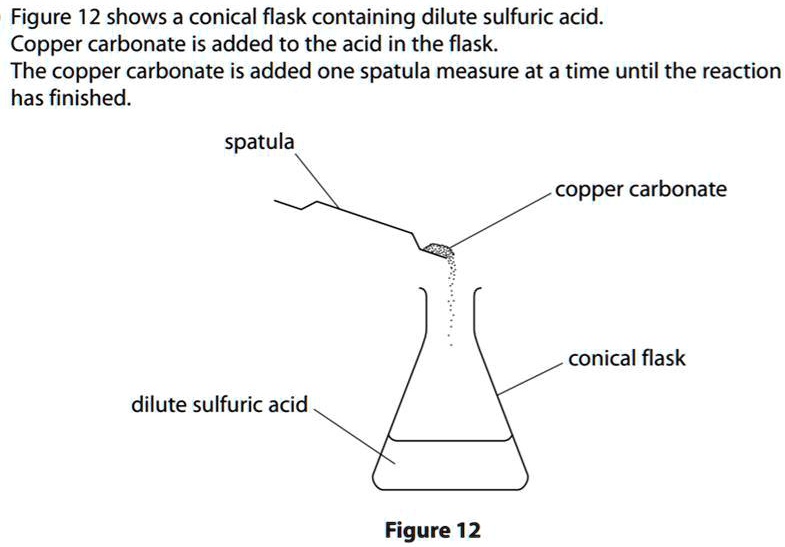
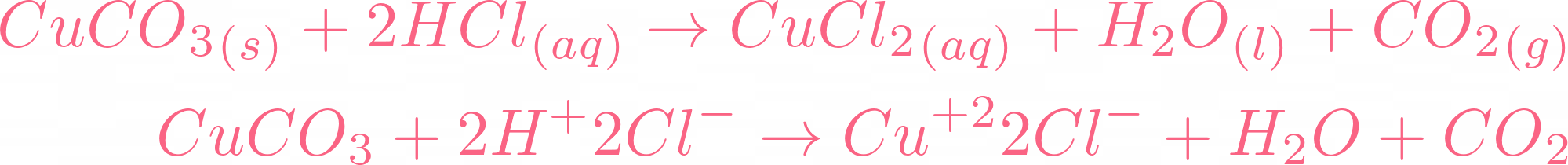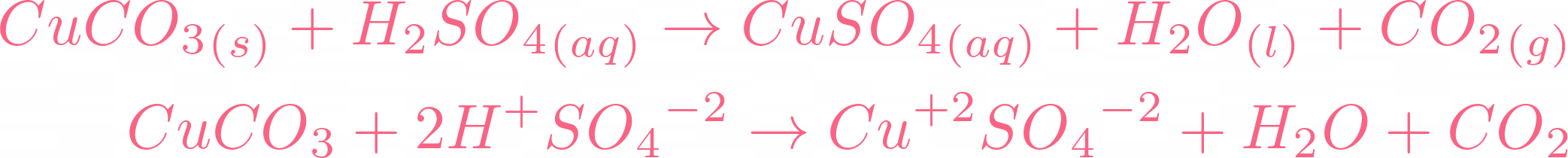Write word equations and then balanced equations for the reaction taking place when:(a) Dilute sulphuric acid reacts with zinc granules.(b) Dilute hydrochloric acid reacts with magnesium ribbon.(c) Dilute sulphuric acid reacts with
![Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition](https://icandochemistry942105908.files.wordpress.com/2021/05/mq2-3.jpg?w=640)
Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition

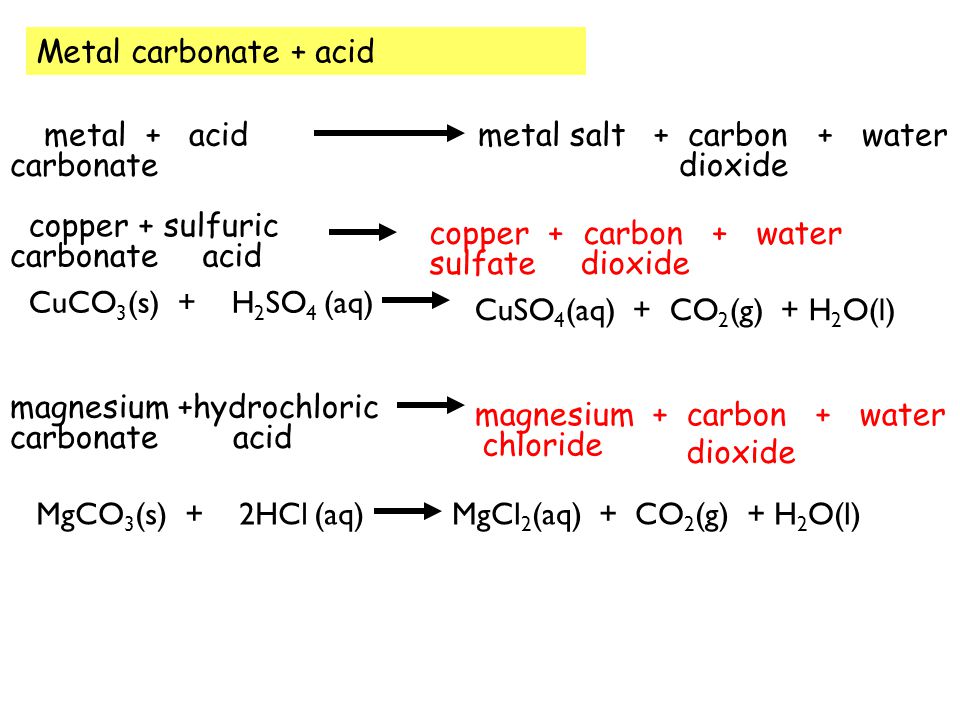
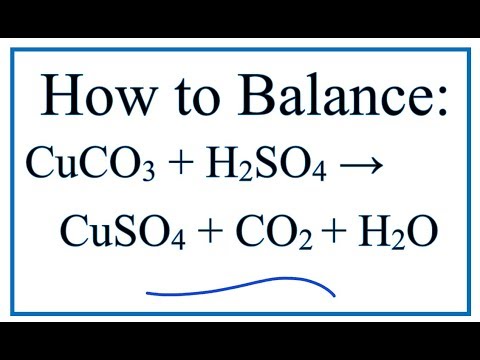
Choosing the substances from the list given below, write balanced chemical equations for the reaction which would be used in the laboratory to obtain the following salts. [Dilute sulphuric acid, Copper, Copper(II)
1. Last week's homework - mark your work. 2. This week's note – copy or print and glue into notes jotter. 3. This week's

Most carbonates are insoluble (can not be dissolved in water) except those containing sodium or potassium ions. - ppt download
i) Lead sulphate from lead carbonate (ii) Sodium sulphate using dilute sulphuric acid. (iii) Copper chloride using copper carbonate. - Sarthaks eConnect | Largest Online Education Community