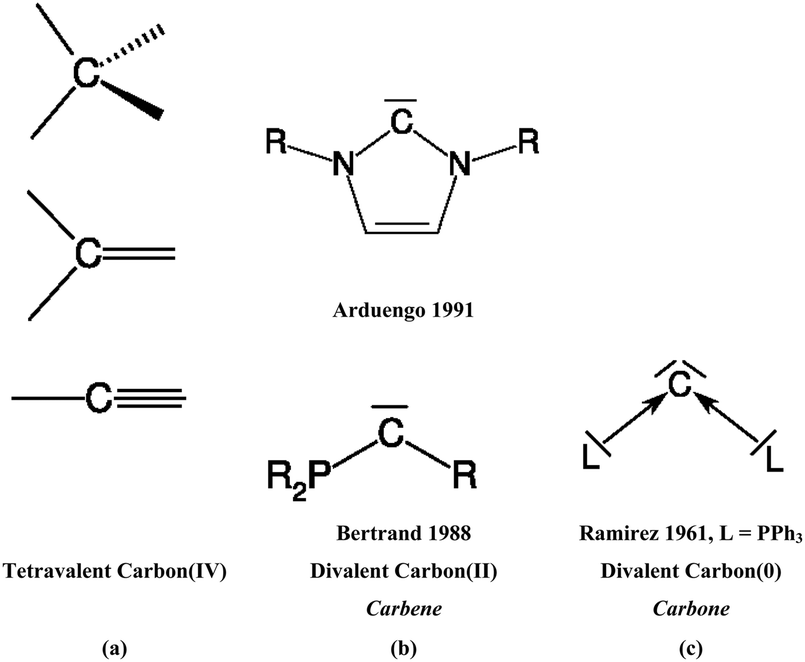
New bonding modes of carbon and heavier group 14 atoms Si–Pb - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C4CS00073K
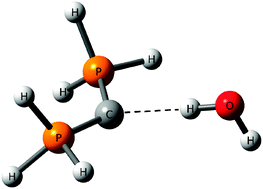
Divalent carbon atom as the proton acceptor in hydrogen bonding - Physical Chemistry Chemical Physics (RSC Publishing)
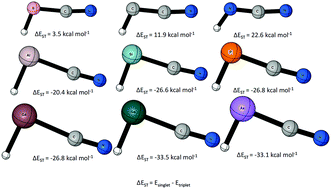
Relatives of cyanomethylene: replacement of the divalent carbon by B−, N+, Al−, Si, P+, Ga−, Ge, and As+ - Physical Chemistry Chemical Physics (RSC Publishing)

Capacitive Deionization of Divalent Cations for Water Softening Using Functionalized Carbon Electrodes | ACS Omega

Structural parameters including bond lengths (Å), divalent bond angles... | Download Scientific Diagram

A neutral divalent carbon species obtained by the removal of two monovalent atoms from the same carbon is called a

![Q-1-1-52E Divalent carbon species called c... [FREE SOLUTION] | StudySmarter Q-1-1-52E Divalent carbon species called c... [FREE SOLUTION] | StudySmarter](https://studysmarter-mediafiles.s3.amazonaws.com/media/textbook-exercise-images/image_8qiN6Fq.png?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIA4OLDUDE42UZHAIET%2F20230529%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20230529T210003Z&X-Amz-Expires=90000&X-Amz-SignedHeaders=host&X-Amz-Signature=c9895e10940de7c36e0dd37623a87b8cf27a8fccaaae5100161b0af405b5b612)

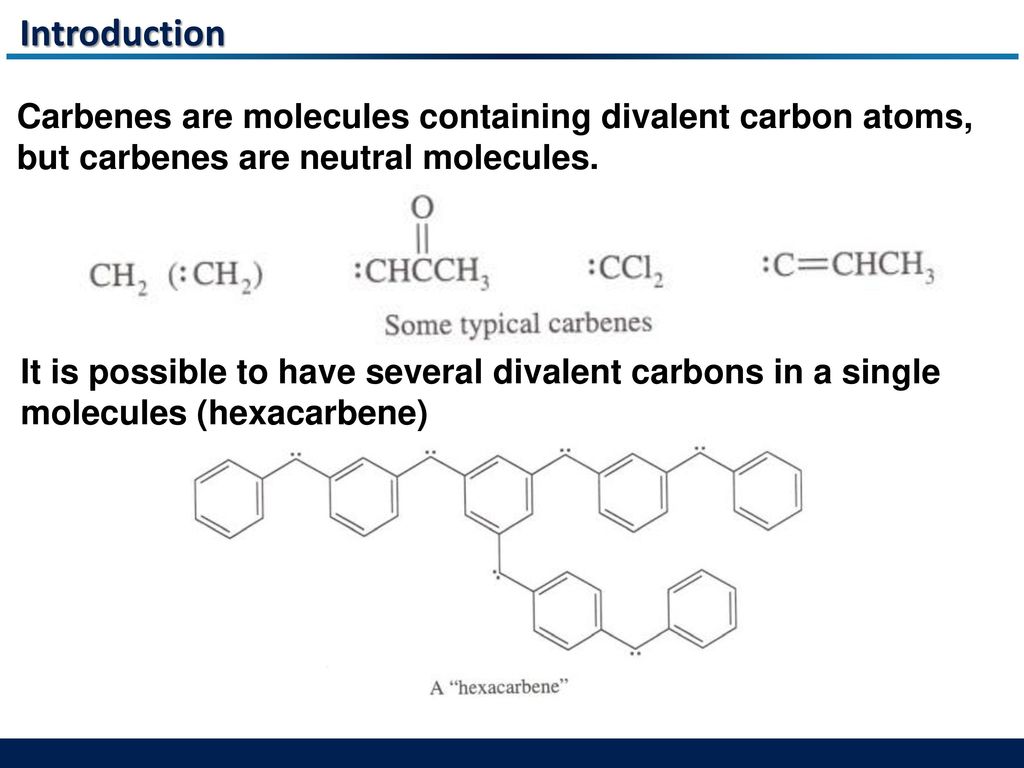


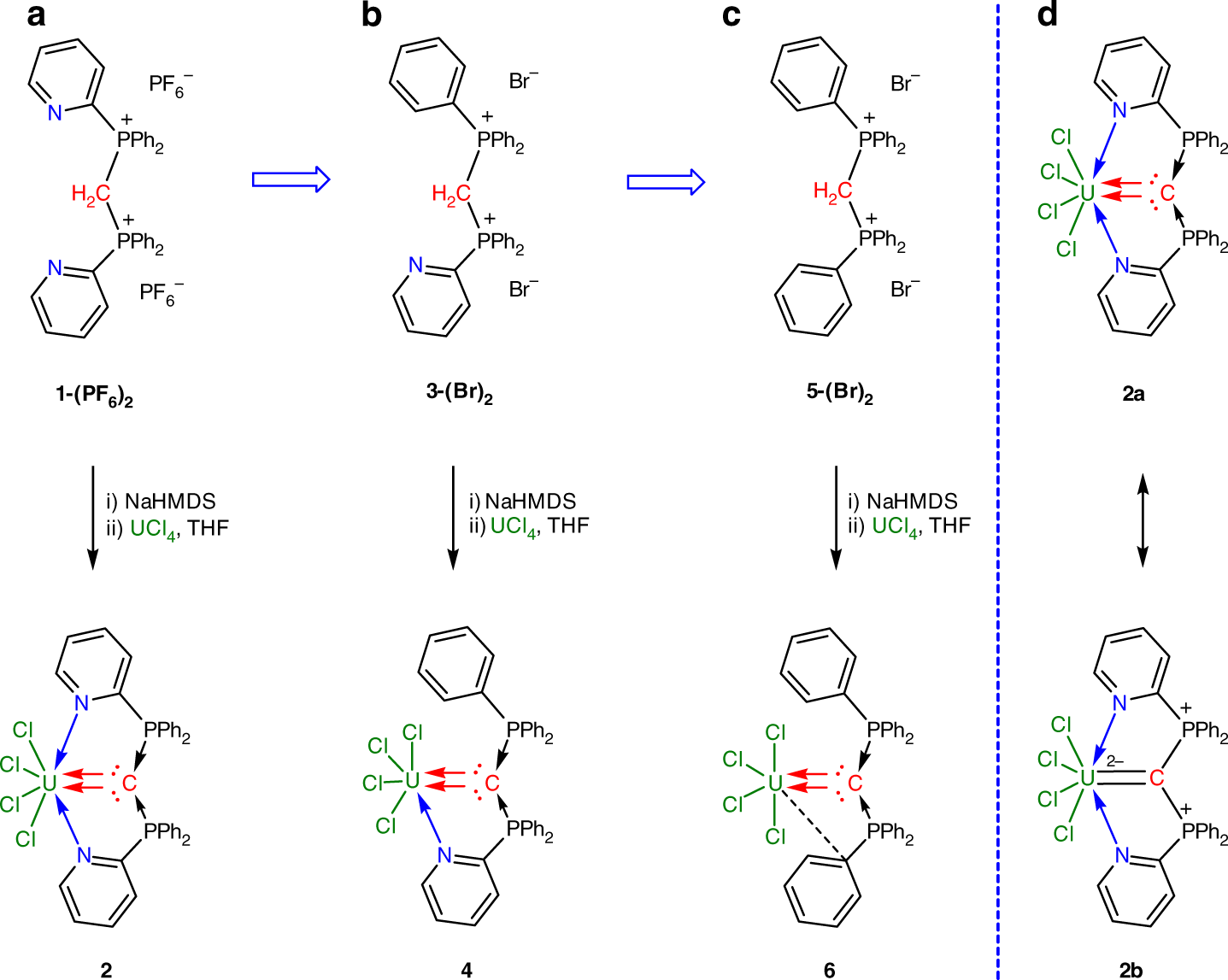

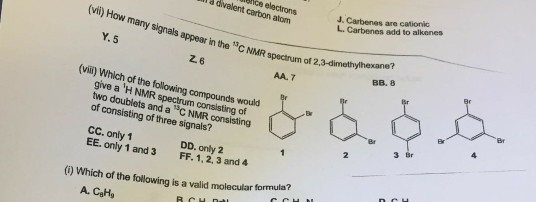
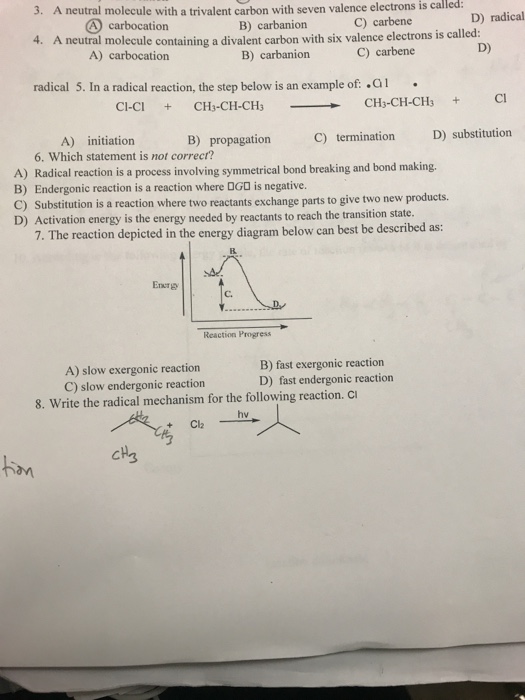

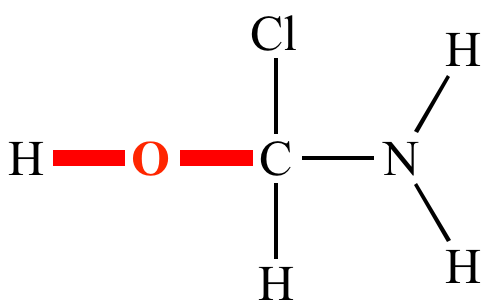



![PDF] Divalent carbon(0) compounds | Semantic Scholar PDF] Divalent carbon(0) compounds | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/d81c5855cec844c13dcc00b1aaa659c91c729854/4-Table1-1.png)