A hydrocarbon contains 82.8% of carbon. Find its molecular formula if its vapour density is 29 [H = 1, C = 12] - Sarthaks eConnect | Largest Online Education Community

Chemical Formulae, Equations, Calculations | Edexcel IGCSE Chemistry: Combined Science Paper 1 2019 (Hard) | Save My Exams
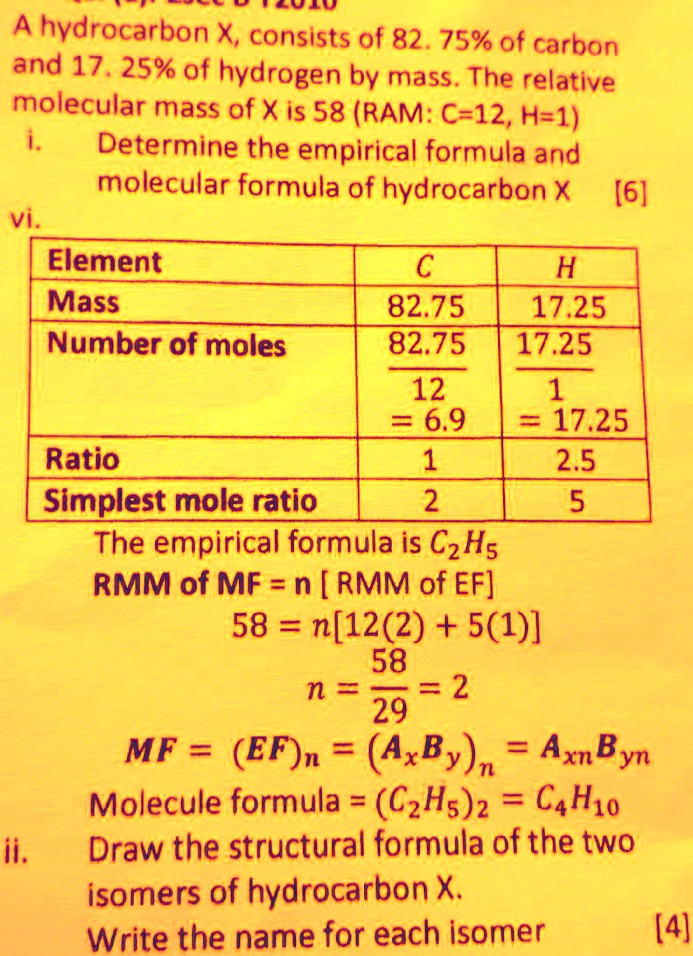
SOLVED: LEOed A hydrocarbon X, consists of 82.75% of carbon and 17. 25% of hydrogen by mass. The relative molecular mass of X is 58 (RAM: C-12,H-1) Determine the empirical formula and

Volatile Organic Compounds and Carbonyls Pollution in Mexico City and an Urban Industrialized Area of Central Mexico - Aerosol and Air Quality Research

An alkane contains 82.8% carbon and 17.2% hydrogen by mass. Show by calculation that the empirical formula - Brainly.in

Simple Synthesis of a Library of Zwitterionic Surfactants via Michael-Type Addition of Methacrylate and Alkane Thiol Compounds | Langmuir
![A hydrocarbon contains 82.8% of carbon. Its molecular formula if its vapour density is 29 is : [H = 1, C = 12] A hydrocarbon contains 82.8% of carbon. Its molecular formula if its vapour density is 29 is : [H = 1, C = 12]](https://dwes9vv9u0550.cloudfront.net/images/4364361/37e45132-5f74-4434-b04b-63051a70a186.jpg)
A hydrocarbon contains 82.8% of carbon. Its molecular formula if its vapour density is 29 is : [H = 1, C = 12]

Chemical Formulae, Equations, Calculations | Edexcel IGCSE Chemistry: Combined Science Paper 1 2019 (Hard) | Save My Exams
What are the emperical formulae and molecular formulae for a compound with 84% carbon and 16% hydrogen if its molecular weight is 400g? - Quora
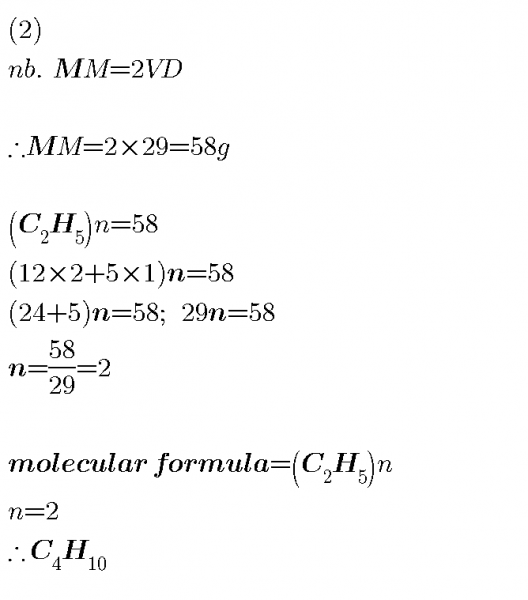
A gaseous hydrocarbon contain 82.75% carbon by mass.Given that the vapour density of the gas... - Myschool
![A hydrocarbon contains 82.8% of carbon. Its molecular formula if its vapour density is 29 is : [H = 1, C = 12] A hydrocarbon contains 82.8% of carbon. Its molecular formula if its vapour density is 29 is : [H = 1, C = 12]](https://dwes9vv9u0550.cloudfront.net/images/8613707/34a23bdf-f946-4801-bf3a-e8894a7348e3.jpg)
A hydrocarbon contains 82.8% of carbon. Its molecular formula if its vapour density is 29 is : [H = 1, C = 12]

A hydrocarbon contains 82.8% of carbon. Find its molecular formula if its vapour density is 29(H = 1, C = - Brainly.in