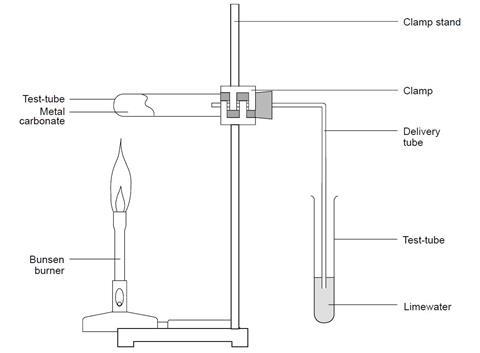
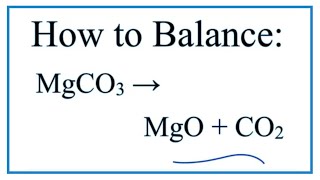
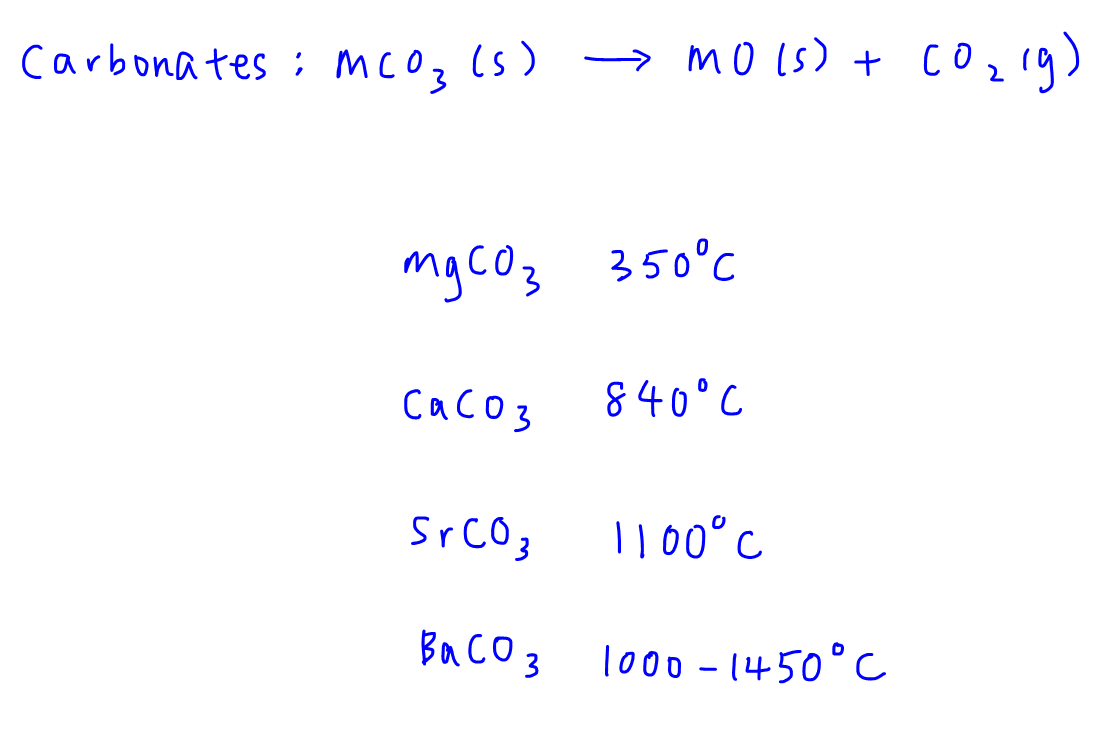20.0 g of a magnesium carbonate sample decomposes on heating to give carbondioxide and 8.0g of magnesium oxide.what will be the percentage purity of magnesium carbonate in thje sampl
20.0 g of a magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 g magnesium oxide. - Sarthaks eConnect | Largest Online Education Community

Metal carbonates are known to undergo thermal decomposition, producing the metal oxide and releasing carbon dioxide. The process is described by the following generic equation, in which M represents an unknown divalent
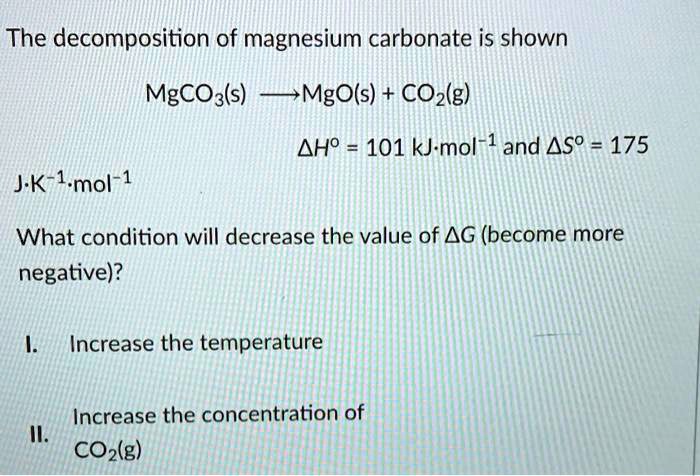
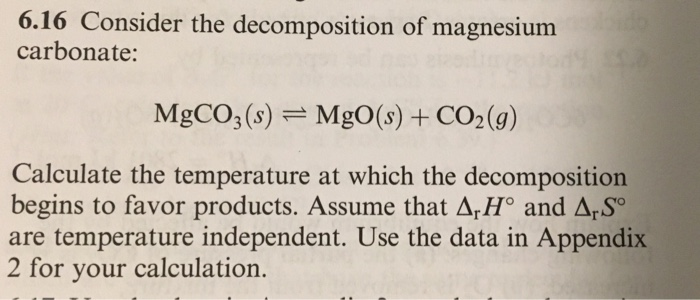
SOLVED: The decomposition of magnesium carbonate is shown MgCOz(s) MgO(s) COz(g) AHo 101 kJ-mol 1 and Aso = 175 JK-1 mol-1 What condition will decrease the value of AG (become more negative)?

20 g of magnesium carbonate sample decomposes on heating to give carbon dioxide and 8 g of Mg..... - YouTube

TGA/SDTA thermal decomposition data of MgCO3.xH2O (Red plot: sample... | Download Scientific Diagram

20.0 g of a magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 g - YouTube

Answer the questions below based on the following decomposition reaction at 25 oC: MgCO3 (s) ? MgO (s) + CO2 (g) Table showing Delta Hf, Delta S and Delta Gf values for

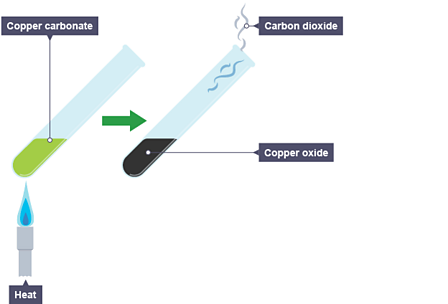
OB: All about decomposition reactions Decomposition reactions are the opposite of synthesis, where a larger reactant is broken down into 2 or more smaller. - ppt download

20.0 mg of a magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 mg magnesium oxide. What will be the percentage purity of magnesium carbonate in the sample? [Atomic
Calculate the percentage composition of the elements present in magnesium carbonate. How many kilogram of CO2 - Sarthaks eConnect | Largest Online Education Community

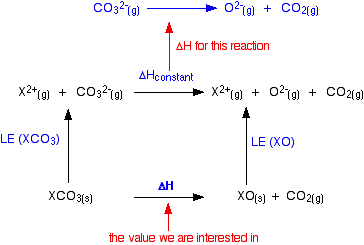
Scheme 1. Thermal decomposition of various MgO precursors: (1) Mg(OH) 2... | Download Scientific Diagram

PROBLEM: Heating some metal carbonates, among them magnesium carbonate, leads to their decomposition. MgCO3(g) → MgO(s) + CO2(g) (a) Calculate. - ppt download